Comparative study of layer by layer assembled multilayer films based on graphene oxide and reduced graphene oxide on flexible polyurethane foam: flame retardant and smoke suppression properties†
Abstract
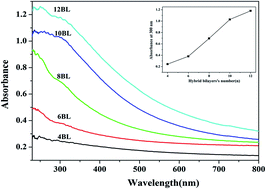
Flame retardant multilayer films based on graphene materials were deposited on the surface of flexible polyurethane (FPU) foam by an advanced layer by layer assembly method (hybrid bilayer approach) in an effort to reduce its flammability. Here, the system of hybrid bilayers was comprised of sodium alginate (SA)/graphene oxide (GO) and polyethylenimine (PEI), in which SA and GO were held together by the combination of electrostatic attraction and hydrogen bonding in a common solution. Firstly, GO coated FPU foams were prepared by alternately submerging FPU foams into SA/GO suspension and PEI solution. Subsequently, reduced graphene oxide (RGO) coated FPU foams were prepared by the thermal reduction of GO based multilayer films at a temperature of 180 °C. The results of thermogravimetric analysis showed that RGO coated FPU foams have higher thermal stability than that of GO coated FPU foams in the temperature range from 430 to 600 °C. The results of cone calorimeter testing indicated that all coated FPU foams have lower peak heat release rate, peak smoke production rate and total smoke production compared with that of the pristine FPU foam. The “delayed effect” in heat release rate and smoke production rate was observed for coated FPU foams with high bilayers number (>3), revealing that graphene material based multilayer films have excellent physical barrier effect. The comparative study in GO and RGO coated FPU foams indicated that the fire safety properties of GO coated FPU became worse after the thermal reduction, which can be ascribed to the loss of a greater amount RGO layers and the reduced thickness of the LbL coating.

 Please wait while we load your content...
Please wait while we load your content...