Polyvinylidene fluoride hollow fiber mixed matrix membrane contactor incorporating modified ZSM-5 zeolite for carbon dioxide absorption
Abstract
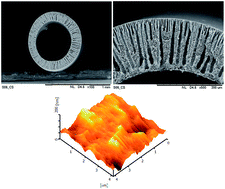
ZSM-5 (Zeolite Socony Mobil-5) was modified using hexadecyltrichlorosilane (HDTS) to increase its hydrophobicity. The modified ZSM-5 was applied in different concentrations (0, 0.25, 0.5 and 1 wt% of solution) in the polyvinylidene fluoride (PVDF) (18 wt% of solution) spinning dope to fabricate hollow fiber mixed matrix membranes for CO2 absorption in a gas–liquid membrane contactor. The properties of the fabricated membranes, including gas permeance, mechanical stability and wetting resistance, were examined. The morphologies of the membrane structures and surface roughness were studied using SEM and AFM, respectively. The SEM images showed that the HFM structure was changed from sponge-like to finger-like with big macrovoids. The AFM analysis showed that the outer surface roughness of the membrane was increased by increasing the ZSM-5 concentration in the spinning dope. By the addition of ZSM-5 zeolite to the polymer, the dope gas absorption performance of the fabricated membranes was improved. The optimum ZSM-5 concentration of 0.5 wt% of solution for the gas–liquid contacting process was obtained. The maximum absorption flux of 1.65 × 10−3 mol m−2 s−1 was achieved at a liquid flow rate of 300 ml min−1 for the composite membrane fabricated using the optimum amount of ZSM-5, which was about 100% more than the plain PVDF membrane flux.

 Please wait while we load your content...
Please wait while we load your content...