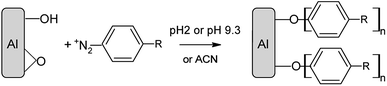
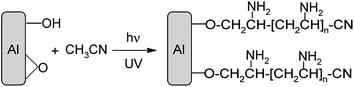
Grafting of an aluminium surface with organic layers†
Avni Berishaac,
Hassan Hazimehb,
Anouk Galtayriesd,
Philippe Decorsea,
Frédéric Kanoufiab,
Catherine Combellasab,
Jean Pinson*a and
Fetah I. Podvorica*ac
aSorbonne Paris Cité, Univ Paris Diderot, ITODYS, UMR 7086 CNRS, 15 rue J-A de Baïf, 75013 Paris, France. E-mail: fetah.podvorica@uni-pr.edu; jean.pinson@univ.paris-diderot.fr
bPSL Research University, ESPCI ParisTech, 10 rue Vauquelin, 75231 Paris Cedex 05, France
cChemistry Department of Natural Sciences Faculty, University of Prishtina, rr. “Nëna Tereze” Nr. 5, 10000 Prishtina, Kosovo
dPSL Research University, Chimie ParisTech – CNRS, Institut de Recherche de Chimie Paris, 75005 Paris, France
First published on 11th August 2016
Abstract
The grafting of organic films on an aluminum surface with its native oxide is demonstrated in protic or aprotic media by various methods: (i) spontaneous reduction of aryldiazonium salts, (ii) simultaneous electrochemical grafting of diazonium salts and alkyl iodides, (iii) spontaneous reaction of perfluoroalkylamine in an organic solvent and (iv) photochemical grafting of acetonitrile. The films are characterized by IRRAS, XPS, electrochemistry, water contact angles and ToF-SIMS; the latter demonstrates that aryl groups derived from diazonium salts are attached by an O–aryl bond. With all methods, the organic layers are strongly attached to the aluminum surface as they resist ultrasonic cleaning. Superhydrophobic films can be obtained when perfluoroalkyl groups are bonded on the surface.
Introduction
Aluminum is an important and widely used engineering metal that is protected by a native oxide layer (2–10 nm thick).1–4 The functionalization of its surface has gained increased interest in a variety of applications. For example, anodising,5 powder coating or wet coating permit to enhance different properties of aluminum such as its visual appearance, its excellent corrosion resistance and its wear resistance. Different classes of organic molecules allow its surface modification such as fatty acids,6,7 phosphonic acids8–11 and silanes.12,13 The spontaneous grafting of aluminum plates14,15 and nanoparticles16 by diazonium salts in acetonitrile has also been reported. The stability of the organic film on the aluminum surface depends strongly on the interaction between the oxidized metallic surface terminated by hydroxyl groups17–19 and the organic moieties.In the case of fatty acids, there is an electrostatic interaction between the charged metal surface and anions derived from the organic molecules. In this way, a roughened and porous aluminum surface can be modified with the hydrophobic C18 alkyl chains from stearic acid to give a super hydrophobic surface.20
With phosphonic acids and silanes, chemical bonds are formed between surface O atoms and P or Si atoms, respectively. When the organic film attached on the aluminum surface contains reactive groups, further chemical modifications are possible. For example, an Al surface modified with [12-(4-benzophenone)-dodecyl]phosphonic acid,10 can be further transformed with different polymers, by reaction of the attached benzophenone moieties, under UV irradiation, with the spin-cast polymer film. In this way, it is possible to tune the hydrophobicity of the Al surface by choosing the appropriate polymer.10 A simple procedure to obtain highly hydrophobic surfaces is achieved in one step by dipping an aluminum substrate in a NaOH solution of a fluorinated silane.12 However, a drawback of these methods is the possible hydrolysis of Si–O and P–O bonds in a basic medium.
The films formed during electrografting or spontaneous grafting of aryldiazonium salts on metals evidence a high stability due to the formation of a covalent bond between a C atom of the aromatic moiety and the metal surface.21–23 These grafting reactions can be performed in an aprotic medium (for example acetonitrile –ACN–) and also in aqueous acidic and basic solutions.24–26 In addition to carbon and metals, diazonium salts have also been reacted with oxide surfaces: ITO,27–30 TiO2,31–33 SnO2,34,35 SiO2,36 MnO2,37 CuO14 and Fe2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 to give metal oxide–(Ar)n surfaces. Aluminum nanoparticles have also been modified by spontaneous reaction with alkyl iodides in toluene39 and with diazonium salts in ACN; in the latter case both Al–aryl and Al–O–aryl bonds were detected by ToF-SIMS.16
38 to give metal oxide–(Ar)n surfaces. Aluminum nanoparticles have also been modified by spontaneous reaction with alkyl iodides in toluene39 and with diazonium salts in ACN; in the latter case both Al–aryl and Al–O–aryl bonds were detected by ToF-SIMS.16
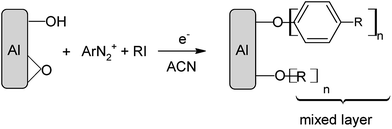
More recently, it was shown on glassy carbon and gold that the reduction of an aryl diazonium ArN2+ in the presence of an alkyliodide RI leads to the formation of (i) alkyl surfaces if the aryl radical cannot bind to the surface (because of steric hindrance) or, (ii) mixed aryl/alkyl surfaces metal–[Ar + R]. The reaction mechanism involves the formation of an aryl radical, abstraction of an iodine atom from the alkyl iodide to give an alkyl radical and grafting of both radicals on the surface.40,41 This method permits to modulate the surface properties of metals by a proper choice of the two groups and of their relative concentrations.
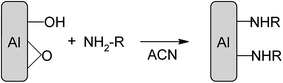
Amines can be grafted on carbon and Pt surfaces by electrochemistry through an intermediate aminyl radical42,43 to give metal–NHR surfaces, and on metallic oxides the same surface is obtained spontaneously through a nucleophilic substitution44 of a metal–OH group.
ACN, a very common solvent, permits the modification of metallic surfaces either indirectly by electrochemical reduction of the 2,6-dimethylbenzenediazonium salt45 or under UV irradiation.46 In both cases, cyanomethyl radicals are the key-species that give organic films such as metal–[CH2–CH(CN)]n.
Herein, different modifications of the surface of aluminum are described: (i) spontaneous reaction of diazonium salts in acidic or basic aqueous media and also in ACN, (ii) electrochemical reduction of diazonium salts in the presence of an iodo alkane in ACN, (iii) spontaneous reaction of amines in ACN and, (iv) photochemical activation of ACN. Different types of reactions are involved: electron transfer, nucleophilic substitution and photochemical homolytic cleavage. The grafted layers present different surface functional groups that permit to adjust their surface chemistry and their hydrophilicity/phobicity. They are characterized by IRRAS, XPS, water angle measurements and ToF-SIMS.
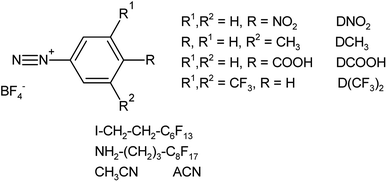
The chemicals used to modify the aluminum surface are diazonium salts (4-nitro, 3-methyl, 3,5-bis trifluoromethyl and 4-carboxybenzenediazonium tetrafluoroborate salts), along with the iodoalkane and the amine and acetonitrile; their structure is presented in Scheme 1.
Experimental
Chemicals
ACN Chromasolv (99.9%) was from Across Organics; acetone, 4-nitrobenzenediazonium tetrafluoroborate (DNO2), hydrochloric acid (37%) and sodium hydroxide were from Sigma-Aldrich. Milli-Q water (>18 MΩ) was used for rinsing the plates and for the preparation of solutions. 1-Iodo-1H,1H,2H,2H-perfluorooctane (ICH2CH2C6F13, RFI) 97% was purchased from Lancaster. 4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-Heptadecafluoroundecylamine (H2N(CH2)3C8F17, RFNH2) 97% was purchased from Aldrich. 4-Carboxybenzenediazonium (DCOOH) and 3,5-(bis)-trifluoromethylbenzenediazonium (D(CF3)2) were prepared by standard procedures.10Aluminum samples
Massive industrial aluminum discs (1 cm diameter) were polished using 0.04 μm alumina slurry on DP-NAP polishing cloth with a Mecatech 234 polishing machine (Presi). After the polishing step, they were rinsed with Milli-Q water, sonicated for 10 min in acetone, and dried under a stream of nitrogen. They were stored under ambient conditions before their modification. In some experiments, they were etched for 5 min in 0.1 M NaOH to roughen the surface.Electrografting
These experiments were performed with an EG&G 263A potentiostat/galvanostat and an Echem 4.30 version software. The reference electrode was Ag/AgCl and the counter electrode a platinum foil. Prior to the analysis, the modified discs were rinsed with copious amounts of water and sonicated in acetone for 6 or 8 min.Photografting
Photochemical modifications were achieved via a wide spectrum UV irradiation mercury Pen Ray lamp UVPC-86079 (20 mA AC, 4400 μW cm−2 at 254 nm for a distance of ∼2 cm) with emission at 184.9 (3%, ∼150 μW cm−2), 253.6 (100%), 312.5–313.1, 356.0–356.3, 407.7 and 435.8 nm. Prior to irradiation, a thin film of neat ACN was deposited onto the metallic discs that were placed 4 cm under the UV light source. After UV irradiation, the discs were rinsed with ACN, acetone and ultrasonicated for 10 min in acetone.Spontaneous modification
To obtain a roughened surface, some Al discs were treated for 5 min in 0.1 M aqueous NaOH solution, rinsed with acetone for 8 min in an ultrasonic bath and left for 2 h in a 10 mM solution of the diazonium salt either in (i) a 0.01 M H2SO4 solution, (ii) a buffer solution at pH = 9.3 (Na2CO3/NaHCO3) or, in (iii) ACN. In acidic solution, the surface becomes yellow, but this colour disappears upon sonication in ACN and DMF. The same procedure was used with a 15 mM RFNH2 solution in ACN. Prior to the analysis, the modified discs were rinsed with copious amounts of water and sonicated in acetone for 8 min.IRRAS instrumentation
Spectra of modified aluminum discs were recorded using a purged (low CO2, dry air) Jasco FTIR-6100 Fourier Transform Infra Red Spectrometer equipped with MCT (mercury–cadmium–telluride) detector. For each spectrum, 1000 scans were accumulated with a spectral resolution of 4 cm−1. The background recorded before each spectrum was that of a clean substrate. In the case of the modification in acidic or basic media the backgrounds were registered after the same treatment.XPS instrumentation
X-ray photoelectron spectra were recorded using a Thermo VG Scientific ESCALAB 250 system fitted with a micro-focused, monochromatic Al Kα X-ray source (1486.6 eV) and a magnetic lens, which increases the electron acceptance angle and hence the sensitivity. The pass energy was set at 100 and 40 eV for the survey and the narrow regions, respectively. The Avantage software, version 4.67, was used for digital acquisition and data processing. The spectra were calibrated against C1s set at 285 eV.Water contact angles
They were measured with a Kruss DSA3 instrument. A drop (3 μL) of Milli-Q water was automatically deposited on the top of the test sample placed in a horizontal position on the instrument stage. At least five measurements were made for each sample. The values of the contact angles were calculated by the tangent method using DropShapeAnalysis software.ToF-SIMS
The spectra were acquired using a TOF-SIMS V spectrometer (IONTOF GmbH). The analysis chamber was maintained at less than 5 × 10−7 Pa under operating conditions. The total primary ion flux was below 1012 ions cm2 to ensure static conditions. A pulsed 25 keV Bi+ primary ion source (Liquid Metal Ion Gun, LMIG) at a current of about 1 pA (high current bunched mode), rastered over a scan area of 500 × 500 μm, was used as the analysis beam.Results and discussion
The reactions scrutinized here involve radicals in solution on Al surfaces or nucleophilic attack of amines. As well documented in the literature, the surface of Al is constituted of Al–OH groups and Al–O oxides,17–19 which both should react with radicals while only Al–OH groups would react with amines.Spontaneous modification with diazonium salts in aqueous acidic, basic and ACN solutions
In an aqueous acidic medium and in aprotic non-nucleophilic solvents, Ar–N2+ cations are present,47 but at neutral and basic pHs, equilibria between Ar–N2+ and the diazohydroxide, Ar–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NOH, or the diazoate, Ar–N
NOH, or the diazoate, Ar–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O−, are established; the pKas for the reaction of water on various Ar–N2+ have been determined.48 The dediazonation of diazonium salts occurs either homolytically or heterolytically; the homolytic cleavage gives a radical that reacts with the surface.49
N–O−, are established; the pKas for the reaction of water on various Ar–N2+ have been determined.48 The dediazonation of diazonium salts occurs either homolytically or heterolytically; the homolytic cleavage gives a radical that reacts with the surface.49
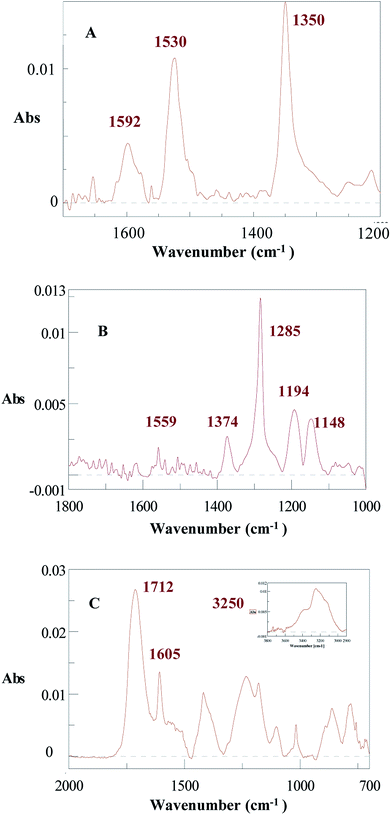 | ||
| Fig. 2 IRRAS spectra of Al discs after spontaneous modification in 0.01 M H2SO4 for 1 h by (A) DNO2, (B) D(CF3)2 and (C) DCOOH. Ultrasonic cleaning for 8 min in acetone. | ||
The grafting of an aluminum surface was also performed with D(CF3)2 in an aqueous acidic medium. The IR spectrum of the modified surface (Fig. 2B) exhibits the medium intensity vibrations of the aromatic ring at 1559 cm−1, a very strong band at 1275 cm−1 and medium ones at 1374, 1194, 1148 cm−1 due to stretching vibrations of the C–F bonds (by comparison: 1598, 1355, 1276, 1206, 1139 cm−1 for D(CF3)2). These bands confirm the presence of 3,5-bis-trifluoromethylphenyl moieties on the Al surface.
An Al surface was also modified with 10 mM DCOOH (formed in situ from the corresponding amine) in an aqueous acidic medium. The IR spectrum of the modified surface (Fig. 2C) presents a broad band in the 3000–3500 cm−1 region with a peak at 3250 cm−1 attributed to –OH stretching (insert in Fig. 2C), a strong band at 1712 cm−1 due to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and a band at 1605 cm−1 assigned to the aromatic ring puckering (3000–3500, 1720, 1615 cm−1 for DCOOH).
O stretching and a band at 1605 cm−1 assigned to the aromatic ring puckering (3000–3500, 1720, 1615 cm−1 for DCOOH).
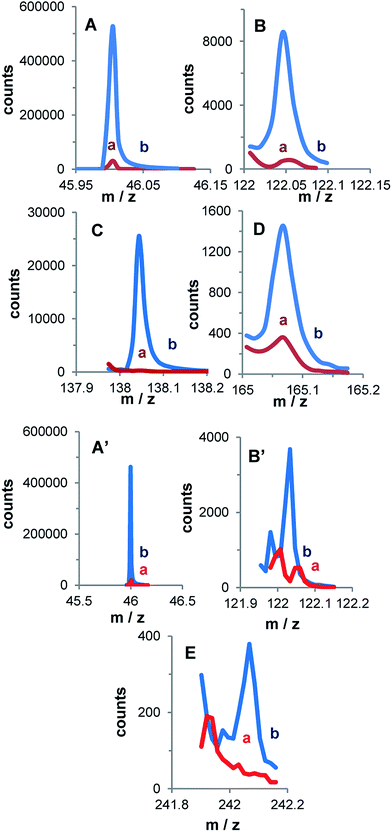
The first disc presents the signals of NO2−, C6H4NO2− and also of O–C6H4NO2− and Al–O–C6H4NO2−. The latter two fragments indicate that the organic film is bonded through an oxygen atom to the alumina surface. There is no signal that would indicate a direct bonding of the film to an Al atom, contrary to what was observed with Al nanoparticles where part of the aryl groups was directly bonded to Al.38 The difference originates from the preparation conditions of the Al samples: nanoparticles were prepared and modified in an oxygen free glove box, while the discs were handled in air.
When the oxidized aluminum surface has been maintained for 1 h in the acidic solution of the diazonium salt, aside from the NO2− and C6H4NO2− fragments, the C6H3NO2–C6H3NO2− peak corresponding to the formation of a multilayer is observed. Such fragments have already been observed after spontaneous or electrochemical modification of a carbon surface.57 However, the coverage of the surface is only partial as fragments pertaining to surface oxides are still obtained.
The coupling of aryl radicals derived from diazonium salts to metal oxide surfaces forming an O–Ar bond has already been observed14,36,37 and the growth of the layer results, as previously proposed on metals, from the attack of aryl radicals on the first grafted aryl layer.55 On the surface of oxidized Al (partly Al–OH and partly Al2O3), the coupling can take place either by H abstraction by the aryl radical or direct attack on the oxygen of Al2O3 (Scheme 2).
Electrochemical modification with diazonium salts in the presence of alkyl halides
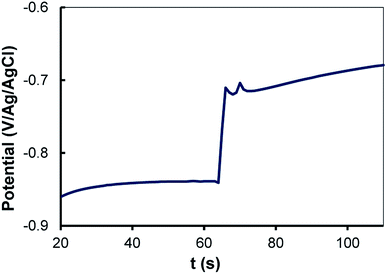
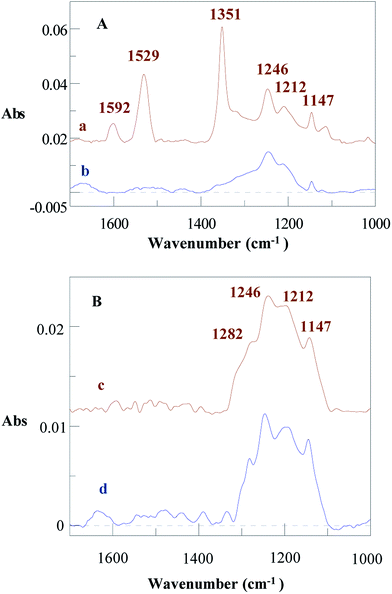
On an Au surface in ACN + 0.1 M NBu4BF4, the reduction of RFI takes place at a quite negative potential, Ep = −2.0 V/Ag/AgCl. Such a potential is at the negative limit of the activity domain of an Al electrode in this medium, which precludes recording a voltammogram of this species. We have previously shown that it is possible to reduce alkyl halides with a large electrocatalytic effect reaching up to 2.1 V and simultaneously attach alkyl groups to carbon and metal surfaces. This has been achieved by reducing diazonium salts at potentials between +0.2 and −0.6 V per SCE, forming an aryl radical that abstracts an iodine atom and furnishes an alkyl radical that finally reacts with the surface.39,40 Mixed surfaces were then formed by grafting at the same time an aryl radical from the diazonium salt and an alkyl radical from the alkyl halide to the surface and the first grafted layer.Following the same process, DNO2 (40 mM) was reduced electrochemically in the presence of RFI (200 mM) by chronoamperometry at E = −0.8 V/Ag/AgCl for 30 min in an ACN + 0.1 M NBu4BF4 solution. At this potential, only the diazonium salt is reduced. However, the IRRAS spectrum of the modified Al disc (Fig. 5a) presents the signature of the perfluoroalkyl groups at 1246, 1212 and 1147 cm−1 (1236, 1206, 1144 cm−1 for I(CH2)2C6F13) and of the 4-nitrophenyl group at 1351 and 1529 cm−1 (symmetric and asymmetric NO2 stretching) and 1592 cm−1 (ring vibration). With DCH3, the peaks of the perfluoroalkyl groups are located at the same wavenumbers as above (Fig. 5b). The water contact angle of the film obtained from DNO2 in the presence of I(CH2)2C6F13 is Θ = 116 ± 3°, indicating a highly hydrophobic film.
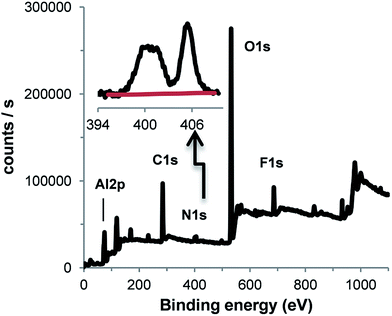
To increase the rugosity of the Al surface prior to any functionalization reaction, an Al disc was treated with 0.1 M NaOH for 5 min. Then, D(CF3)2 (40 mM) was reduced in the presence of RFI (200 mM) in ACN by chronoamperometry at E = −0.8 V per SCE for 15 min on such an Al disc. The IRRAS spectrum of the modified surface (Fig. 5c) presents the signal of aryl–CF3 group at 1282 cm−1 and the perfluoroalkyl bands at 1246, 1212 and 1147 cm−1. The XPS survey spectrum (Fig. ESI. 1†) shows the contributions of Al2p (74.9 eV, 20.6%), C1s (285.0 eV, 25.0%), N1s (400.2 eV, 1.0%), O1s (532.2 eV, 46.8%) and F1s (685.6 eV, 6.6%). The Al2p peak, which corresponds to the oxidized form only, can be deconvoluted into two contributions: 74.9 eV (17.8%) for Al2O3 + Al–OH17,18,51 and 75.6 eV (2.33%) for AlF3 (75.80 eV (ref. 50)). C1s presents a number of contributions in agreement with the complex structure of such films40 among which CF3 (293.6 eV) is clearly identified by comparison with the XPS spectrum of RFI grafted on GC (293.7 eV39) and that of trifluoromethylbenzene (293.8 eV50). F1s can be deconvoluted in two peaks at 685.6 (5.2%) for AlF3, 3H2O and 688.8 eV (1.2%) for the perfluoroalkyl group (689.7 eV for polytetrafluoroethylene59 and 690.8 eV (ref. 60) for C6H5CF3). In order to find out if the presence of AlF3, 3H2O could be due to the reaction of the perfluoroalkyl compound with aluminum, perfluorooctane was deposited on the surface of Al and left for 30 min; after rinsing, XPS does not show any increase of the AlF3 contribution (not shown).
Using both perfluorinated alkyl and trifluoromethyphenyl groups, the surface becomes superhydrophobic with a water contact angle Θ = 161 ± 3° (Fig. ESI. 2†).
The stability of these films was tested by washing out the modified roughened plate for 4 h with toluene in a Soxhlet. The Al disc modified film by the perfluoroalkyl chains and the 3,5-bis trifluoromethylphenyl groups only shows a small decrease of the IRRAS intensity (less than 5% at 1246 cm−1, Fig. 5d), but the spectrum is better defined than that before washing, indicating the removal of some adsorbed species during the extraction with boiling toluene.
Upon one electron reduction on glassy carbon, the diazonium salt is reduced to an aryl radical that abstracts a iodine atom from RI to give a R˙ radical.39 This permits to reduce the alkyl iodide with a huge electrocatalytic effect (∼2.1 V in the case of DNO2). On the Al discs, the potential was maintained at −0.8 V/Ag/AgCl to ensure the reduction of the diazonium salt to a radical prone to iodine atom abstraction from the alkyl iodide. Therefore, both aryl and alkyl radicals should be obtained as on glassy carbon and both attach to the Al surface. The structure of the obtained multilayers should be analogous to that already observed on glassy carbon (Scheme 3).39
Spontaneous modification with amines
The two spontaneous grafting reactions reported above rely on the reduction of diazonium salts by an electron transfer from Al through the surface oxide film. We now report a surface modification that is not based on an electron transfer but on a nucleophilic substitution of hydroxyl groups. An Al disc treated previously with a 0.1 M NaOH aqueous solution for 5 min to increase the rugosity of the surface was thereafter immersed for 2 h into a solution of ACN containing 15 mM RFNH2. The IRRAS spectrum of the modified Al disc (Fig. 6) presents the NH deformation peak at 1583 cm−1 and the CF stretching peaks at 1248, 1217 and 1152 cm−1.61 The XPS survey spectrum (Fig. ESI. 3†) shows the contribution of Al2p (74.7 eV, 23.4%), C1s (284.9–293.6 eV), N1s (400.2 eV, 1.3%), O1s (532.3 eV, 34.9%) and F1s (685.6 eV, 21.6%). Al2p can be deconvoluted in three peaks at 71.6 (1.75%), 74.6 (16.0%) and 75.4 eV (5.4%) corresponding respectively to Al, Al2O3 + Al–OH and AlF3 as above. The C1s peak can be deconvoluted with a peak at 293.6 eV corresponding to the terminal CF3.40 The 400.2 eV N1s peak is close to the 398.8 eV reported value for the N–metal bond on iron and copper.43 F1s can be deconvoluted in two peaks at 685.4 eV (16.0%) and 688.8 eV (5.6%) corresponding respectively to AlF3, 3H2O as above and the perfluoroalkyl group. The water contact angle is Θ = 110 ± 2°.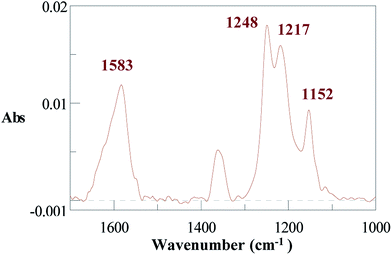 | ||
| Fig. 6 IRRAS spectrum for an Al disc treated in an aqueous basic solution and immersed for 2 h into an ACN + 15 mM H2NC2H4C8F17 solution. Ultrasonic cleaning for 8 min in acetone. | ||
The attack of amines on copper or iron surface oxides is reported to involve the nucleophilic substitution of a M–OH group (M = metal) by RNH2 to give M–NHR.43 We assume that the same mechanism takes place on Al where OH groups constitute a high fraction of the surface.17 In organic chemistry, unprotonated hydroxyl are poor leaving groups, but Al is much more electropositive than carbon (electronegativity 1.63 vs. 2.55), which increases the polarity of the bond and favors the cleavage and substitution of the OH group (Scheme 4).
Photochemical modification with acetonitrile
The IRRAS spectrum of an Al disc immersed in ACN and irradiated with UV light for 45 min (Fig. 7a) presents the same features as those obtained when gold, copper, nickel and iron samples are modified under photochemical irradiation of ACN.45 The two strong bands at ∼3250 and ∼1630 cm−1 correspond to –NH2 stretching and –NH2 deformation respectively and the band at ∼1400 cm−1 is attributed to –CH2 scissoring vibrations. These bands are not present when the Al disc has been maintained for the same time in ACN without irradiation (Fig. 7b). This IR spectrum indicates the modification of the surface by amino groups.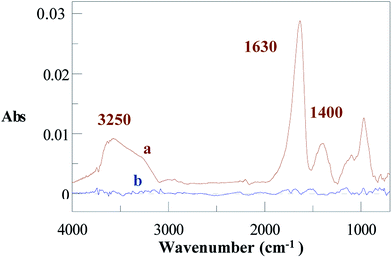 | ||
| Fig. 7 IRRAS spectra of Al discs immersed in ACN for 45 min (a) under irradiation or (b) without irradiation. Ultrasonic cleaning for 8 min in acetone. | ||
The reaction is triggered by the very reactive cyanomethyl radical, ˙CH2CN obtained by irradiation of ACN (that strongly absorbs below 190 nm). This radical abstracts a hydrogen atom from the hydroxylated surface; the coupling of the oxygen and cyanomethyl radicals provides a bonded Al–O–CH2CN group. The growth of the layer can follow two different paths involving either the ˙CH2CN radical or the –CH2CN anion44,45 that should be produced by reduction of ˙CH2CN. Because of the values of the radical/carbanion couple (E°(˙CH2CN/−CH2CN) = −0.66 V/Ag/AgCl)62 and of the ocp of the Al native oxide surface in ACN (Eocp(AlACN) = −0.75 V/Ag/AgCl) are close, both routes are possible (Scheme 5).
Conclusion
Four different approaches are presented for the modification of the Al native oxide surface by (i) spontaneous grafting of aryl diazonium salts (prepared ex or in situ) in aqueous acidic or basic media and in ACN; (ii) electrografting of an alkyl iodide in the presence of a diazonium salt, leading to mixed alkyl/aryl films; (iii) spontaneous grafting of amines; (iv) photografting of acetonitrile that gives aminated surfaces. Owing to (ii), the proper choice of the two starting compounds permits to obtain superhydrophobic surfaces. Different mechanisms are involved: reaction of radicals on the oxidized Al surface (aryl, perfluoroalkyl and ˙CH2CN radicals) or nucleophilic attack of Al by amines. The radicals are obtained either by spontaneous or electrochemical reduction of diazonium salts. The layers characterized by IRRAS and XPS are strongly attached to the Al surface as they resist sustained ultrasonic cleaning. The aryl groups (obtained from diazonium salts) are bonded to the oxidized Al surface via an oxygen atom, as evidenced by Al–O–Ar fragments in ToF-SIMS experiments. These methods are very simple and quite general; they use easily available starting compounds and should therefore find specific applications in the surface modification of Al.References
- J. R. Davis, Aluminum and Aluminum Alloys, ASM International, 1993 Search PubMed.
- E. Ghali, Corrosion resistance of aluminum and magnesium alloys, Wiley, Hoboken, 2010 Search PubMed.
- R. Strohmeier, Surf. Interface Anal., 1990, 15, 51 CrossRef.
- M. R. Alexander, G. E. Thompson, X. Zhou, G. Beamson and N. Fairley, Surf. Interface Anal., 2002, 34, 485 CrossRef CAS.
- W. Lee and S.-J. Park, Chem. Rev., 2014, 114, 7487 CrossRef CAS PubMed.
- I. Liascukiene, N. Aissaoui, S. J. Asadauskas, J. Landoulsi and J.-F. Lambert, Langmuir, 2012, 28, 5116 CrossRef CAS PubMed.
- K. Nielsch, J. Choi, K. Schwirn, R. B. Wehrspohn and U. Gösele, Nano Lett., 2002, 2, 677 CrossRef CAS.
- M. J. Pellerite, T. D. Dunbar, L. D. Boardman and E. J. Wood, J. Phys. Chem. B, 2003, 107, 11726 CrossRef CAS.
- J. Pahnke and J. Rühe, Macromol. Rapid Commun., 2004, 25, 1396 CrossRef CAS.
- M. Giza, P. Thissen and G. Grundmeier, Langmuir, 2008, 24, 8688 CrossRef CAS PubMed.
- C. Schmitt Pauly, A.-C. Genix, J. G. Alauzun, M. Sztucki, J. Oberdisse and P. H. Mutin, Phys. Chem. Chem. Phys., 2015, 17, 19173 RSC.
- N. Saleema, D. Sarkar, R. Paynter and X. Chen, ACS Appl. Mater. Interfaces, 2010, 2, 2500 CAS.
- N. Saleema, D. K. Sarkar, D. Gallant, R. W. Paynter and X.-G. Chen, ACS Appl. Mater. Interfaces, 2011, 3, 4775 CAS.
- B. L. Hurley and R. L. McCreery, J. Electrochem. Soc., 2004, 151, B252 CrossRef CAS.
- A. M. Mahmoud, A. J. Bergren and R. L. McCreery, Anal. Chem., 2009, 81, 6972 CrossRef CAS PubMed.
- Y. Aït Atmane, L. Sicard, A. Lamouri, J. Pinson, M. Sicard, C. Masson, S. Nowak, P. Decorse, J.-Y. Piquemal, A. Galtayries and C. Mangeney, J. Phys. Chem. C, 2013, 117, 26000 Search PubMed.
- E. McCaferty and J. P. Wightman, Surf. Interface Anal., 1998, 26, 549 CrossRef.
- J. van den Brand, P. C. Snijders, W. G. Sloof, H. Terryn and J. H. W. de Wit, J. Phys. Chem. B, 2004, 108, 6017–6024 CrossRef CAS.
- Ö. Özkanat, B. Salgin, M. Rohwerder, J. M. C. Mol, J. H. W. de Wit and H. Terryn, J. Phys. Chem. C, 2012, 116, 1805 Search PubMed.
- L. Feng, H. Zhang, P. Mao, Y. Wang and Y. Ge, Appl. Surf. Sci., 2011, 257, 3959 CrossRef CAS.
- Aryl Diazonium Salts. New Coupling Agents in Polymer and Surface Science, ed. M. M. Chehimi, Wiley-VCH, Weinheim, 2012 Search PubMed.
- D. Bélanger and J. Pinson, Chem. Soc. Rev., 2011, 40, 3995 RSC.
- J. Pinson and F. Podvorica, Chem. Soc. Rev., 2005, 34, 329 RSC.
- C. Combellas, M. Delamar, F. Kanoufi, J. Pinson and F. I. Podvorica, Chem. Mater., 2005, 17, 3968 CrossRef CAS.
- F. I. Podvorica, F. Kanoufi, J. Pinson and C. Combellas, Electrochim. Acta, 2009, 54, 2164 CrossRef CAS.
- A. Berisha, F. Kanoufi, J. Pinson, C. Combellas and F. I. Podvorica, Electrochim. Acta, 2011, 56, 10762 CrossRef CAS.
- S. Maldonado, T. J. Smith, R. D. Williams, S. Morin, E. Barton and K. J. Stevenson, Langmuir, 2006, 22, 2884 CrossRef CAS PubMed.
- A.-M. Haque and J. K. Kim, Langmuir, 2011, 27, 882 CrossRef CAS PubMed.
- A.-M. Haque and J. K. Kim, Chem. Commun., 2011, 47, 6855 RSC.
- F. Mirkhalaf, T. J. Mason, D. J. Morgan and V. Saez, Langmuir, 2011, 27, 1853 CrossRef CAS PubMed.
- A. Merson, T. Dittrich, Y. Zidon, J. Rappich and Y. Shapira, Appl. Phys. Lett., 2004, 85, 1075 CrossRef CAS.
- A. Mesnage, M. Abdel Magied, P. Simon, N. Herlin-Boime, P. Jégou, G. Deniau and S. Palacin, J. Mater. Sci., 2011, 46, 6332 CrossRef CAS.
- D. Quinton, A. Galtayries, F. Prima and S. Griveau, Surf. Coat. Technol., 2012, 206, 2302 CrossRef CAS.
- A. Adenier, N. Barré, E. Cabet-Deliry, A. Chaussé, S. Griveau, F. Mercier, J. Pinson and C. Vautrin-Ul, Surf. Sci., 2006, 600, 4801 CrossRef CAS.
- F. Lamberti, S. Agnoli, L. Brigo, G. Granozzi, M. Giomo and N. Elvassore, ACS Appl. Mater. Interfaces, 2014, 6, 22769 CAS.
- C. Bureau and J. Pinson, Procédé de modification de surfaces isolantes ou semi-conductrices, et produits tels qu'obtenus, European patent, EP1948720, 2006.
- K. J. Bell, P. A. Brooksby, M. I. J. Polson and A. J. Downard, Chem. Commun., 2014, 50, 13687 RSC.
- K. Brymor, J. Fouineau, A. Eddarir, F. Chau, N. Yaacoub, J.-M. Grenèche, J. Pinson, S. Ammar and F. Calvayrac, J. Nanopart. Res., 2015, 17, 438 CrossRef.
- M. Fogliazza, L. Sicard, P. Decorse, A. Chevillot-Biraud, C. Mangeney and J. Pinson, Langmuir, 2015, 31, 6092 CrossRef CAS PubMed.
- D. Hetemi, F. Kanoufi, C. Combellas, J. Pinson and F. I. Podvorica, Langmuir, 2014, 30, 13907 CrossRef CAS PubMed.
- D. Hetemi, H. Hazimeh, P. Decorse, A. Galtayries, C. Combellas, F. Kanoufi, J. Pinson and F. I. Podvorica, Langmuir, 2015, 31, 5406 CrossRef CAS PubMed.
- B. Barbier, J. Pinson, G. Desarmot and M. Sanchez, J. Electrochem. Soc., 1990, 101, 1757 CrossRef.
- A. Adenier, M. M. Chehimi, I. Gallardo, J. Pinson and N. Vilà, Langmuir, 2004, 20, 8243 CrossRef CAS PubMed.
- I. Gallardo, J. Pinson and N. Vilà, J. Phys. Chem. B, 2006, 110, 19521 CrossRef CAS PubMed.
- A. Berisha, C. Combellas, F. Kanoufi, J. Pinson, S. Ustaze and F. I. Podvorica, Chem. Mater., 2010, 22, 2962 CrossRef CAS.
- A. Berisha, C. Combellas, G. Hallais, F. Kanoufi, J. Pinson and F. I. Podvorica, Chem. Mater., 2011, 23, 3449 CrossRef CAS.
- C. Galli, Chem. Rev., 1988, 88, 765 CrossRef CAS.
- A. Sienkiewicz, M. Szymula, J. Narkiewicz-Michaleka and J. Bravo-Díaz, J. Phys. Org. Chem., 2014, 27, 284 CrossRef CAS.
- R. Pazo-Llorente, H. Maskill, C. Bravo-Díaz and E. Gonzalez-Romero, Eur. J. Org. Chem., 2006, 2201 CrossRef CAS.
- D. Mercier, M. Herinx and M. G. Barthés-Labrousse, Corros. Sci., 2010, 52, 3406 CrossRef.
- F. Le Floch, J.-P. Simonato and G. Bidan, Electrochim. Acta, 2009, 54, 3078 CrossRef CAS.
- J. Lehr, B. E. Williamson, B. S. Flavel and A. J. Downard, Langmuir, 2009, 25, 13503 CrossRef CAS PubMed.
- NIST X-ray Photoelectron Spectroscopy Database. NIST Standard Reference Database 20, Version 4.1, data compiled and evaluated by A. V. Naumkin, A. Kraut-Vass, S. W. Gaarenstroom and C. J. Powell Search PubMed.
- J. A. Treverton and N. C. Davies, Electrochim. Acta, 1980, 25, 1571 CrossRef CAS.
- A. Adenier, E. Cabet-Deliry, A. Chaussé, S. Griveau, F. Mercier, J. Pinson and C. Vautrin-Ul, Grafting of Nitrophenyl Groups on Carbon and Metallic Surfaces Without Electrochemical Induction, Chem. Mater., 2005, 17, 491–501 CrossRef CAS.
- P. Doppelt, G. Hallais, J. Pinson, F. Podvorica and S. Verneyre, Chem. Mater., 2007, 19, 4570–4575 CrossRef CAS.
- C. Combellas, F. Kanoufi, J. Pinson and F. I. Podvorica, Langmuir, 2005, 21, 280 CrossRef CAS PubMed.
- L. Li, J. Li, X. Du, A. Welle, M. Grunze, O. Trapp and P. A. Levkin, Angew. Chem., Int. Ed., 2014, 53, 3835 CrossRef CAS PubMed.
- High resolution XPS of organic polymers. The Scienta ESCA300 Database, ed. G. Beamson and D. Briggs, John Wiley, Chichester, UK, 1992 Search PubMed.
- D. T. Clark, D. Kilcast and W. K. R. Musgrave, J. Chem. Soc. D, 1971, 516 RSC.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies, John Wiley, New York, 3rd edn, 2001 Search PubMed.
- A. A. Isse and A. Gennaro, J. Phys. Chem. A, 2004, 108, 4180 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15313e |
| This journal is © The Royal Society of Chemistry 2016 |