Effects of oxygen content in the atmosphere on thermal oxidative stabilization of polyacrylonitrile fibers
Abstract
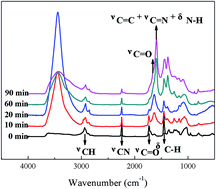
The effects of oxygen content in the atmosphere on thermal oxidative stabilization of polyacrylonitrile fibers have been studied based on the evolution of the interaction between cyclization and oxidation reactions. The results indicate that cyclization propagation was inhibited in the initial few minutes due to radical adsorption by the oxygen in the atmosphere, and then the cyclization was promoted with dehydrogenation going by oxidation, whereas the oxidation reaction was always promoted with the cyclization structure. Resultantly, a high oxygen content atmosphere didn't result in a low cyclization degree and excessive oxidation in stabilized fibers, while a low oxygen content atmosphere introduced a skin–core structure because of insufficient oxygen diffusion and rapid oxidation rate in the later stage. Finally, according to the tensile strength of carbon fibers, the optimum oxygen content in the thermal stabilization atmosphere was determined to be 15–21%.

 Please wait while we load your content...
Please wait while we load your content...