Electrospinning applications from diagnosis to treatment of diabetes
Abstract
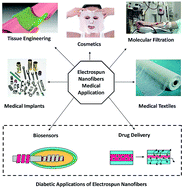
Electrospinning is a facile, yet low cost and reproducible technique that can use both natural and synthetic polymers to address problems in diagnosis and treatment of diabetes. For the diagnosis of diabetes, effective continuous glucose monitoring of the blood glucose level can be achieved by using electrospun glucose biosensors. Electrospun nanofibers confer a high-surface area, micro-porosity, and potential to encapsulate drugs or biomolecules within nanofibers. Even though electrospinning has been used widely there is no review available till now with the applications of electrospinning specifically for the diagnosis and treatment of diabetes. In this critical review, recent advances of electrospinning to optimize the glucose sensing ability and a myriad of diabetic drug delivery techniques via electrospinning are discussed. Future perspectives of biodegradable nanofibers are also discussed in the last section, which highlights the current challenges, innovation and development of novel electrospun nanofibers for theranostics targeted to diabetics.

 Please wait while we load your content...
Please wait while we load your content...