Morphology effect of polythiophene catalysts on photo-degradation of methylene blue†
Yue Yua,
Jiancheng Jina,
Xi Yua,
Xiangheng Xiaob and
Xinlin Hong*a
aCollege of Chemistry and Molecular Sciences, Wuhan University, Wuhan, P. R. China. E-mail: hongxl@whu.edu.cn; Fax: +86 27 68756619; Tel: +86 27 68756619
bSchool of Physics and Technology, Wuhan University, Wuhan, P. R. China
First published on 1st August 2016
Abstract
Polythiophene (PTh) nanosheets and spheres were successfully synthesized by changing the oxidation ability of ferric ions without any templates. Their distinct morphologies caused by different growth processes lead to different photo-catalytic activity for methylene blue (MB) degradation under visible light. The mechanism of morphology–performance relationship was thoroughly studied. It shows that different morphologies are the reflection of different length and bond-connection of PTh chains, which are the real reasons for different performance.
In the past decades, as one kind of important conductive polymer, polythiophene (PTh) has attracted much attention in electrochemistry,1–3 biomedical engineering4 and catalysis5 because of its excellent chemical and physical properties, such as big π-conjugation polymeric chains, moderate band gap, good environment stability and electrical conductivity. PTh itself has been utilized by B. Muktha as a photo-catalyst for degradation of various textile dyes in UV radiation.6 And the major method developed in these years to improve the photocatalytic property of PTh is to modulate the PTh particles with metals, oxides, graphene, and some other additives.7–10 However, there is little work focused on regulating the catalytic performance of PTh by adjusting its morphology, even though many different morphologies of PTh, including sphere,11,12 film,13,14 ribbon,12,15 nanotube and nanofiber12,16–18 have been reported in the past. As we know, the morphology of catalyst has much to do with its surface or interface, which is the real place where the catalytic reaction happens, and which is tighted related to catalytic activity. Therefore, it is an efficient way to adjust the catalytic activities by controlling the morphology of PTh.
In this work, a simple template-free method was developed to prepare PTh materials with different morphologies such as spheres with different size and nanosheet. Among them, the PTh nanosheet has not been reported before. These PTh materials with different morphologies show different photo-catalytic activity in degradation of MB under visible light. In details, PTh nanosheet shows the strongest adsorption ability of MB and the best photo-catalytic activity, while the PTh sphere with smaller size has the strongest absorption for visible light. And the degradation rate constant of PTh nanosheet is 3.6 and 13.3 times higher than that of smaller PTh sphere and bigger PTh sphere, respectively. These investigations on the morphology–performance relationship of conductive polymer catalysts can help the rational design of excellent catalysts for this important environmental catalytic process.
The detailed preparation procedures of PTh materials with different morphologies were listed in supporting information, and the smaller PTh sphere (Fig. 1a), the bigger PTh sphere (Fig. 1b) and the PTh nanosheet (Fig. 1c) were named PTh-1, PTh-2 and PTh-3 respectively. The obtained PTh materials were utilized as catalysts for photo-degradation of MB, which was conducted as follows: 0.1 g catalyst were put into a beaker containing 100 mL MB solution (20 mg L−1), the solution was stirred in the dark for 2 h to reach adsorption equilibrium and then 0.25 mL H2O2 was added, the solution was irradiated using a 4.2 W LED light with CREE XM-L2 lamp. The relative radiation power at different wavelength of LED light is shown in Fig. S1, ESI.† The samples were taken at set intervals, and its concentration of MB was detected using UV-vis spectrum after centrifugation. The photo-catalytic data are shown in Fig. 1d, and their apparent rate constants are 0.0934 h−1 (PTh-1), 0.0250 h−1 (PTh-2), 0.3323 h−1 (PTh-3), respectively.
 | ||
| Fig. 1 SEM images of (a) PTh-1, (b) PTh-2, (c) PTh-3. (d) The photo-catalytic kinetics of PThs and the blank under lights. | ||
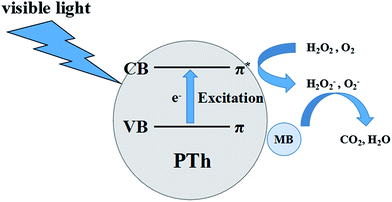
According to the classical photo-catalytic mechanism,7,19–22 the photo-degradation process of MB mainly consists of three steps: (1) adsorption of MB by PTh; (2) excitation of PTh by light and production of electrons to attack H2O2 or O2 to form H2O2−, O2−; (3) oxidation and degradation of MB by H2O2−, O2−. The detailed scheme of the photo-degradation of MB by PTh is shown in Fig. 2. Thus, the ability of MB adsorption and light absorption are both very important. The ability of MB adsorption was investigated carefully first. The percentage of MB adsorption when reaching adsorption equilibrium in the dark after 2 h and the BET surface areas of samples are listed in Fig. 3a. It shows that the MB adsorption ability of platelike PTh-3 (25.0%) is almost ten times larger than that of spherical PTh-1 and PTh-2 (2.5% and 2.6% respectively), not proportional to the changes in BET surface areas of the three samples (26.01 m2 g−1, 12.07 m2 g−1 and 71.00 m2 g−1 for PTh-1, PTh-2 and PTh-3 respectively). This means there should be other factors other than the surface areas of the materials that strongly influenced the MB adsorption.
 | ||
| Fig. 3 (a) MB adsorption and specific surface area, (b) XRD patterns, (c) FTIR spectra and (d) UV-vis spectra of PThs. | ||
Obviously, the microstructure of the adsorbent surface is important for chemisorption. XRD and FTIR were used to character their crystalline structures and molecular structures. XRD characterization (Fig. 3b) indicates that PTh-1 has a similar structure with PTh-2, while PTh-3 shows much more ordered crystalline structure. There are four strong peaks at 2θ = 12.30°, 15.26°, 18.28°, 26.68°, and a broad peak at 2θ ≈ 24° in XRD spectrum of PTh-3, which is quite different from those of the normal PTh materials.22–24 Because XRD spectra show some difference but the basic characteristics are same, it indicates that the crystalline structures are not the main factors for their different MB adsorption. FTIR was also utilized to investigate the differences of the three samples (Fig. 3c). It is seen that PTh-3 shows a strong peak at about 1100 cm−1 which can be assigned to C–O stretching vibration, implying that it was seriously oxidized by oxygen. Other peaks at 790 cm−1, 1026 cm−1 and 1108 cm−1, 1290 cm−1, 1630 cm−1 were assigned to C–H out of plane stretching vibration, C–H in plane stretching vibration, C–C stretching vibration, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C characteristic stretching vibration respectively.25 In addition, the peak at 780 cm−1 was the typical absorption of thiophene rings connected with α–α bond which has a higher degree of molecular planarity than that of α–β bond.26 We normalized the peak height at 1630 cm−1 (C
C characteristic stretching vibration respectively.25 In addition, the peak at 780 cm−1 was the typical absorption of thiophene rings connected with α–α bond which has a higher degree of molecular planarity than that of α–β bond.26 We normalized the peak height at 1630 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C characteristic stretching vibration) and compared the peak height at 780 cm−1. It showed that PTh-3 has the highest peak and PTh-1 has the lowest (Fig. 3c). It shows that the PTh with higher degree of molecular planarity can adsorb more MB. As we know, MB molecule also has a big conjugated structure which can have a stronger π–π interaction with the surface of PTh particles having a higher degree of molecular planarity. Thus, PTh-2 can adsorb more MB than PTh-1 even it has a smaller specific surface area. From the above discussion, it can be concluded that the MB adsorption ability of the PTh sample is determined by the degree of molecular planarity and the specific surface area.
C characteristic stretching vibration) and compared the peak height at 780 cm−1. It showed that PTh-3 has the highest peak and PTh-1 has the lowest (Fig. 3c). It shows that the PTh with higher degree of molecular planarity can adsorb more MB. As we know, MB molecule also has a big conjugated structure which can have a stronger π–π interaction with the surface of PTh particles having a higher degree of molecular planarity. Thus, PTh-2 can adsorb more MB than PTh-1 even it has a smaller specific surface area. From the above discussion, it can be concluded that the MB adsorption ability of the PTh sample is determined by the degree of molecular planarity and the specific surface area.
As another important aspect of the MB photo-degradation process, photo-electron producing ability of the catalyst was studied by using UV-vis spectra (Fig. 3d). The results indicated that there were a stronger absorption peak at about 300 nm and a broad band from 400 nm to 600 nm. The former peak is due to the π–π* transition of the short π-conjugated structure in thiophene unit, while the later one was the result of π–π* transition of the long π-conjugated structure in thiophene backbone.27 From Fig. 3d, it is clear that PTh-1 has the strongest absorption at about 600 nm which means it has the longest π-conjugated structure and could absorb more visible light and produce more photo-electrons than others, while PTh-3 was on the contrary. The weak band at 800 nm of PTh-3 that belongs to the near infrared regions27 was caused by the seriously oxidation, which did not appear in the curves of PTh-1 and PTh-2. Furthermore, PTh-1 has the strongest absorption for visible light, which should be the reason why its catalytic property is better than that of PTh-2 even though they have a similar specific surface area. And that it is worse than PTh-3, probably because of its weak MB adsorption, it do not make the best of its photo-electrons. FTIR was utilized to confirm the changes of these PTh catalysts after the MB photo-degradation. The FTIR curve of PTh-1 and PTh-2 (Fig. 4a and b) showed an obvious peak at 1062 cm−1 which was assigned to C–O stretching vibration, implying that they were oxidized after reaction, but PTh-3 was reduced because the C–O peak was obviously weaken, which implied MB may be oxidized and degraded by PTh-3 because of its own oxidization. More interestingly, we found that after reaction the PTh-1 still had a considerable ability to degrade MB when it was kept in the dark (Fig. 4b), while the activity of PTh-2 was very weak. However, PTh-3 almost showed no catalytic activity in the same condition, probably because the short lifetime of the photo-electron or free radical, and they can not be produced without illumination. We conjectured that the radicals could oxidize PTh-1 and PTh-2 just as oxygen oxidized PTh-3, and the oxidized PTh could become a new oxidant to degrade MB and it is reduced to PTh itself. The possible processes is shown as Scheme 1. Because PTh-1 could produce more photo-electrons and had more surplus radicals, it had much stronger catalytic ability in the dark than PTh-2. And PTh-3 did not have surplus radicals because of its weak absorption of light and strong MB adsorption, it was even reduced by itself, so it showed no catalytic ability in the dark after reaction. And because it shows a much slower degradation rate in the dark than the degradation rate of PTh under illumination, we prefer that this reaction is just a secondary reaction when PTh under illumination, and the oxidized PTh is not a effective oxidizing agent to degrade MB comparing to OH radicals.
 | ||
| Fig. 4 FTIR spectra of PTh-1 (a), PTh-2 (b), PTh-3 (c) before and after photo-degradation reaction. (d) The catalytic kinetics of PThs and the blank after photo-degradation reaction. | ||
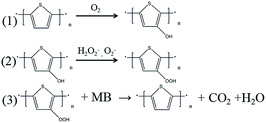 | ||
| Scheme 1 The possible processes of formation of oxidized PTh (1) and degradation MB by oxidized PTh (2). | ||
From the above, it is clear that the MB adsorption, visible light absorption and MB photocatalytic degradation properties of the PTh materials are influenced strongly by their morphologies. And the smaller sphere can absorb most visible light because of its longest chain, and adsorb fewest MB because of its lowest degree of molecular planarity. Obviously, the application of PTh photocatalysts is controlled by the morphology of the materials, which is always determined by its preparation process. Thus the details of the PTh polymerization were carefully investigated in order to find out how different morphologies are formed. SEM images (Fig. S2, ESI†) revealed that the polymerization process of PTh-1 in acetonitrile is much different from that of PTh-2 in the mixture of acetonitrile and chloroform although they both form spheric particles. In acetonitrile, it forms small spheres with some bulges on the surface firstly (Fig. S2a, ESI†). The bulge grows up quickly because of its bigger specific surface area and higher surface energy, and at last it forms spheres in similar size to that of the spheres formed before (Fig. S2b, ESI†). While in the mixture of acetonitrile and chloroform, PTh particle grows randomly like a walnut at first (Fig. S2d, ESI†). Then it grows up and forms a much bigger sphere (Fig. S2g, ESI†). In the system of ethanol and chloroform, many smaller nanosheets can be clearly found on the surface of the bigger nanosheet (Fig. 1c), which suggests that many planes composed by polymer chains overlap each other and form nanosheets. The proposed growing processes of these three PTh materials are shown in Fig. 5.
PTh morphology can be regulated by using different solvents in the polymerization process, and this is due to the solvent-induced difference of polymerization rate, which can be proved by PTh yields in different solvents. The yield of PTh in chloroform and ethanol (2%) is much lower than that in chloroform and acetonitrile (34%), and in acetonitrile (44%), while in pure ethanol, there is almost no product obtained, indicating a much slower polymerization rate than others. Moreover, the reaction is completed in 8 h in acetonitrile, much faster than in acetonitrile and chloroform (24 h) (Fig. S2, ESI†). The solvent-induced difference of polymerization rate may be caused by the electronic properties of the solvent utilized. Acetonitrile has a stronger electron withdrawing group than chloroform, which can enhance the Lewis acid site of the ferric ion28 and enhance its oxidation ability, leading to higher polymerization rate, longer polymer chain and lower degree of molecular planarity. But ethanol is quiet different because of its strong electron donating group, which hinders the polymerization speed. Therefore, adding chloroform to the solvent of acetonitrile slows down the polymerization rate, and slower rate leads to bigger sphere (Fig. S2 and S3, ESI†). When ethanol is introduced in the system, the polymerization is so slow that the monomer has enough time to polymerize according to its own property (strong π–π stacking interaction) to form nanosheets but not sphere (Fig. 1c).
Other factors affecting the polymerization rate were also discussed to find out the decisive factors for controlling the morphology of PTh. It is shown that the concentration of ferric chloride has a marked effect than that of thiophene in acetonitrile. Changing the concentration of monomer (too low or too high with respect to the concentration of ferric ion) only results in the formation of irregular morphology without actually changing the morphology (Fig. S4a and b, 4c and d, ESI†). But it produced nanosheets but not spheres when the concentration of ferric chloride (oxidizing agent) decreased (Fig. S4a, 4c, ESI†), indicating the oxidability-determined morphology control strategy in this preparation process. Similar phenomena can also be found in chloroform and ethanol system, rod and sphere PTh appear when increasing the concentrations of thiophene and ferric chloride are increased at the same time (Fig. S5a–c, ESI†). Additionally, reaction temperature is another important factor influencing the polymerization rate. As shown in Fig. S5a, 5d and 5e, (ESI†), raising the reaction temperature leads to thicker nanosheets, but there was no sphere existed. In PTh-1 and PTh-2 systems, smaller spheric particles can be formed at higher temperatures (Fig. S6, ESI†), indicating that faster polymerization rate lead to smaller spheres again.
In summary, we have successfully synthesized PTh spheres and nanosheet using a template-free method. The morphology of PTh is controlled by the oxidation ability of ferric ion which is strongly affected by its concentration and the electronic property of the solvent. Meanwhile, the monomer concentration and temperature can affect the regularity and size in different ways, but they are not the determinate factors for morphological control. It prefers to form sphere when the oxidation ability of ferric ion is strong. And stronger oxidation ability of ferric ion leads to smaller sphere, longer polymer chain, and lower degree of molecular planarity, and results in stronger absorption of visible light and weaker MB adsorption. What is more, we found that PTh oxidized by oxygen or radicals can also degrade MB. This process is a supplement for the classical photocatalytic process. The present work provides an important method for modulating the microstructure of conductive polymer catalysts, which is useful in the rational design of polymeric photocatalysts in environmental catalysis.
Acknowledgements
This work was financially supported by National Science Foundation of China (NSFC-21373153) and the Fundamental Research Funds for the Central Universities (2042016kf0180).Notes and references
- M. Zhang, X. Guo, Y. Yang, J. Zhang, Z. G. Zhang and Y. Li, Polym. Chem., 2011, 2, 2900 RSC.
- S. L. Ming, S. M. Zhang, H. T. Liu, Y. Zhao, D. Mo and J. K. Xu, Int. J. Electrochem. Sci., 2015, 10, 6598 CAS.
- Y. Kim, S. Cook, S. M. Tuladhar, S. A. Choulis, J. Nelson, J. R. Durrant and M. Ree, Nat. Mater., 2006, 5, 197 CrossRef CAS.
- M. Planellas, M. M. Pérez-Madrigal, L. J. del Valle, S. Kobauri, R. Katsarava, C. Alemán and J. Puiggalí, Polym. Chem., 2015, 6, 925 RSC.
- S. E. Bae, K. J. Kim, Y. K. Hwang and S. Huh, J. Colloid Interface Sci., 2015, 456, 93 CrossRef CAS PubMed.
- B. Muktha, G. Madras, T. N. Guru Row, U. Scherf and S. Patil, J. Phys. Chem. B, 2007, 111, 7994 CrossRef CAS PubMed.
- W. R. Zheng, S. Jones, X. L. Hong and S. C. E. Tsang, RSC Adv., 2014, 4, 47488 RSC.
- F. Zhang, Y. Shi, Z. Zhao, W. Song and Y. Cheng, Appl. Catal., B, 2014, 150, 472 CrossRef.
- M. M. Stylianakis, E. Stratakis, E. Koudoumas, E. Kymakis and S. H. Anastasiadis, ACS Appl. Mater. Interfaces, 2012, 4, 4864 CAS.
- M. R. Chandra, T. S. Rao, S. V. N. Pammi and B. Sreedhar, Mater. Sci. Semicond. Process., 2015, 30, 672 CrossRef.
- L. Ai, Y. Liu, X. Y. Zhang, X. H. Ouyang and Z. Y. Ge, Synth. Met., 2014, 191, 41 CrossRef CAS.
- R. C. Liu and Z. P. Liu, Chin. Sci. Bull., 2009, 54, 2028 CAS.
- P. Ihalainen, M. Pesonen, P. Sund, T. Viitala, A. Määttänen, J. Sarfraz, C. E. Wilén, R. Österbacka and J. Peltonen, Appl. Surf. Sci., 2016, 364, 477 CrossRef CAS.
- S. Chae, K. H. Jo, S. W. Lee, H. S. Keum, H. J. Kim, J. Choi and H. H. Lee, Macromol. Chem. Phys., 2015, 217, 537 CrossRef.
- A. Gök, M. Omastová and A. G. Yavuz, Synth. Met., 2007, 157, 23 CrossRef.
- M. R. Karim, K. T. Lim, C. J. Lee and M. S. Lee, Synth. Met., 2007, 157, 1008 CrossRef CAS.
- M. Fu, Y. Zhu, R. Tan and G. Shi, Adv. Mater., 2001, 13, 1874 CrossRef CAS.
- P. M. DiCarmine, A. Fokina and D. S. Seferos, Chem. Mater., 2011, 23, 3787 CrossRef CAS.
- L. Zhai and R. D. McCullough, J. Mater. Chem., 2004, 14, 141 RSC.
- M. O. Ansari, M. M. Khan, S. A. Ansari and M. H. Cho, J. Saudi Chem. Soc., 2015, 19, 494 CrossRef.
- F. Zhang, Y. J. Shi, Z. S. Zhao, W. J. Song and Y. Cheng, Appl. Catal., B, 2014, 150, 472 CrossRef.
- M. R. Karim, C. J. Lee and M. S. Lee, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5283 CrossRef CAS.
- S. S. Jeon, S. J. Yang, K. J. Lee and S. S. Im, Polymer, 2010, 51, 4069 CrossRef CAS.
- F. Kong, Y. Wang, J. Zhang, H. Xia, B. Zhu, Y. Wang, J. Zhang, H. J. Xia, B. L. Zhu, Y. M. Wang, S. R. Wang and S. H. Wu, J. Mater. Sci. Eng. B, 2008, 150, 6 CrossRef CAS.
- H. Murata, N. Toshima and Y. Saitoh, Bull. Chem. Soc. Jpn., 1993, 140, 244 Search PubMed.
- S. Geetha and D. C. Trivedi, Synth. Met., 2005, 155, 232 CrossRef CAS.
- I. Imae, N. Noma and Y. Shirota, Synth. Met., 1997, 85, 1385 CrossRef CAS.
- D. C. Chen, H. S. Wang, J. P. Wu and X. R. Gu, New Chem. Mater., 1997, 8, 15 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthetic methods and SEM images of PTh synthesized in different conditions. See DOI: 10.1039/c6ra15249j |
| This journal is © The Royal Society of Chemistry 2016 |