Room temperature copper-catalyzed oxidative amidation of terminal alkynes for the synthesis of α-ketoamides using O-benzoyl hydroxylamines as aminating reagent and oxidant†
Abstract
A novel and convenient copper-catalyzed oxidative amidation for the synthesis of α-ketoamides has been successfully developed, which uses easily available O-benzoyl hydroxylamines as aminating reagent and oxidant. The reaction proceeds smoothly at room temperature and is compatible with a range of substrates to give the desired products in moderate to good yields.
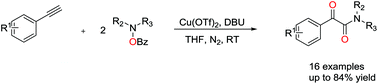

 Please wait while we load your content...
Please wait while we load your content...