Synthesis of nitrogen-functionalized macroporous carbon particles via spray pyrolysis of melamine-resin†
Abstract
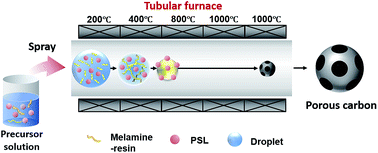
In this study, we developed the first synthesis of macroporous carbon particles with high nitrogen content from a melamine resin via spray pyrolysis. A dual-polymer precursor consisting of melamine resin and a polystyrene latex (PSL) template was used to control the carbon particle morphology. The pore size and porous structure were adjusted by changing the PSL particle size and the PSL/melamine resin ratio, respectively. A PSL/melamine resin ratio of 1.6 : 1 gave the best morphology. Thermal decomposition and carbonization of the melamine resin were performed for several seconds in a tubular furnace. The nitrogen content of the particles obtained at carbonization temperatures between 600 and 1000 °C ranged from 5.44% to 39.2%. The nitrogen content was approximately two to 10 times higher than those achieved using a hydrothermal route. The thermal decomposition was homogeneous and all reactions were performed in droplets, which acted as a micro-reactor system; therefore, we were able to clarify the mechanisms of melamine resin decomposition and particle structuration.

 Please wait while we load your content...
Please wait while we load your content...