Detection of oxidative stress biomarkers in myricetin treated red blood cells
Prabhanshu Kumara,
Nidhee Chaudharya,
Narendra Kumar Sharma b and
Pawan Kumar Maurya*ac
b and
Pawan Kumar Maurya*ac
aAmity Institute of Biotechnology, Amity University Uttar Pradesh, Noida, 201301, India. E-mail: pkmaurya@amity.edu; Fax: +91 120 2432200; Tel: +91 9560869477
bDivision of Infectious Disease, Department of Medicine, Universidade Federal de Sao Paulo – UNIFESP, Brazil
cInterdisciplinary Laboratory for Clinical Neuroscience (LiNC), Department of Psychiatry, Universidade Federal de Sao Paulo – UNIFESP, Brazil
First published on 14th October 2016
Abstract
Homeostasis is a key characteristic of cellular lifespan. Its maintenance influences the rate of aging and age related disorders. Only certain flavonoids have been shown to alter homeostasis of red blood cells in the course of aging. It has been demonstrated that myricetin possesses both antioxidant and pro-oxidant properties. The objective of this study was the determination of the membrane bound oxidative stress biomarkers (Na+, K+-ATPase, Ca2+-ATPase, and Na+, H+ exchanger) activity in myricetin treated red blood cells during human aging. The study was carried out on clinically relevant blood samples obtained from 92 healthy subjects between the ages of 20–79 years. The subjects were divided into three age groups, young (18–35 years), middle (36–60 years) and old (>60 years). The effects of myricetin were evaluated by detecting Na+, K+-ATPase, Ca2+-ATPase, and Na+, H+ exchanger activities by co-incubating the red blood cells in the presence of myricetin (10−8 M to 10−3 M final concentration). The results showed significant (p < 0.001) age dependent decline in the activities of Na+, K+-ATPase, and Ca2+-ATPase and elevation in the activity of Na+, H+ exchanger as compared to the respective young controls. In vitro administration of myricetin significantly attenuated the deleterious effect of oxidative stress in red blood cells from all three age groups. We believe that these findings are novel and they will help in further research against oxidative stress in red blood cells, thereby this study has remarkable scope in medical science.
Introduction
Flavonols belong to the large natural antioxidant group of flavonoids.1 Flavonoids show both anti-oxidative as well as pro-oxidative properties depending on their intracellular concentration. Myricetin (3,5,7,3′, 4′,5′-hexahydroxylflavone) is a natural flavonol, found in many fruits, vegetables, berries, medicinal herbs and other plants.2 It is well known for its nutraceutical value. The chemical uniqueness of myricetin mediates a direct antioxidative effect by the catechol groups in ring B forming a semiquinone radical after oxidation; the 4′-OH group of ring B and 3-OH group forming a quinine methide after oxidation and the keto group in combination with 3-OH or 5-OH group chelating redox active metal ions.3 The motivation behind choosing this flavonoid is its structural characteristic of donating proton that may alter the activity of ion transporters in cell membrane (Fig. 1). Myricetin is highly effective with respect to scavenging of reactive oxygen species (ROS) and provides protection against oxidative stress.4 It has also shown anti-inflammatory5 and anti-mutagenic6 activities. Several polyphenols including myricetin have been reported to increase the life span.7,8 Recently, it has been reported that a methylated derivative of myricetin enhances life span in Caenorhabditis elegans.9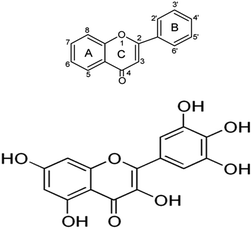 | ||
| Fig. 1 Chemical structure of myricetin. The antioxidant property is due to three OH-groups in ring B. | ||
Aging is a part of natural life cycle of an organism which is linked with morphological, biochemical and functional impairments of the body. Aerobic cells are frequently exposed to free radicals in the form of reactive oxygen species (ROS) and reactive nitrogen species (RNS).10 These free radicals damage biomolecules such as carbohydrates, proteins, lipids, and nucleic acid.11 To provide protection against ROS/RNS, organisms develop endogenous antioxidant defense arrangements that comprise of primary and secondary antioxidants.
Human red blood cells (RBCs) survive in the circulatory system for approximately 115 days. However in practice there is considerable difference in the life span of human erythrocytes, this value may vary between 70 to 140 days.12 Red blood cells membrane consist of several membrane bound enzymes which help in ionic transport across cell membrane.13,14 Ionic transport is essential for the maintenance of viable RBCs in the blood. Several metabolic pathways are involved in the water and solute balance in RBCs and cellular volume is regulated by the monovalent cation concentration.15 The transport of ion across cell membrane is regulated by several enzymes, including Na+, K+-ATPase, Ca2+-ATPase and Na+, H+ exchanger.16,17 Na+, K+-ATPase is a key protein that regulates the cell volume of red blood cells, which is fundamental for avoiding hemolysis, and has huge impact on the deformability of RBCs which is necessary to withstand blood pressure and to pass through narrow vessels.18 The red blood cell Ca2+-ATPase is a highly regulated transporter involved in maintaining Ca2+ homeostasis vital for cellular metabolic function.19 Impairment in ion transporter activity and deregulation of homeostasis has been linked with RBCs membrane fluidity and susceptibility of membrane towards oxidative damage.20,21 Activities of ATPases are modulated by minor changes in the surrounding micro-environment.22 Emerging findings suggest the link between oxidative stress and the ion exchangers.23,24 In recent years, Na+, K+-ATPase has demonstrated significance in oxidative stress related disease states, including obesity, atherosclerosis, heart failure, uremic cardiomyopathy, and hypertension.25 Oxidative stress by ROS at membrane level also disturbs the inherit integrity of ion exchangers which subsequently alters their functions.25–27 Many other metabolic elements such as calcium may alter ion exchangers independently of oxidative stress.28,29 Recently, our group has also established the correlation of NHE activity with Na+, K+-ATPase and Ca2+-ATPase activity with respect to human age.17
Since, there are several questions remaining concern with the antioxidative effect of myricetin in red blood cells, we investigated the effect of myricetin using in vitro red blood cell membrane as a model followed by spectroscopic analysis for detection of oxidative stress biomarkers, Na+, K+-ATPase and Ca2+-ATPase, as shown in Scheme 1.
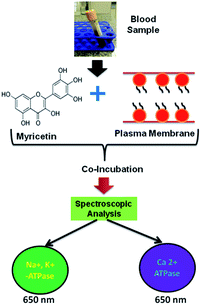 | ||
| Scheme 1 Schematic representation of methodology adapted for analysis of Na+, K+-ATPase, Ca2+-ATPase in blood samples. | ||
Variations in intracellular pH have long been suggested to be important in various metabolic processes. The inactivation of various nutrient transports has been shown previously to influence aging.30 Tightly coupled exchange of Na+ for H+ occurs across the surface membrane of virtually all living cells. Na+, H+ exchanger is ubiquitously expressed in RBCs plasma membrane that plays a crucial role in intracellular pH and cell volume homeostasis by catalyzing an electro-neutral exchange of extracellular sodium and intracellular hydrogen.31 For years, the underlying molecular entity was unknown and the full physiological significance of the exchange process was not appreciated, but much knowledge has been gained in the last two decades. To investigate the effect of myricetin on Na+, H+ exchanger, we used in vitro packed red blood cells (PRBCs) as a model to study proton efflux, as shown in Scheme 2.
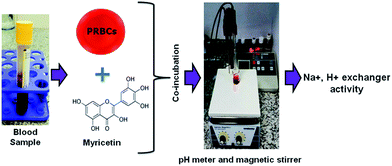 | ||
| Scheme 2 Schematic representation of methodology adapted for analysis of Na+, H+ exchanger in blood sample. PRBCs, packed red blood cells. | ||
Results and discussion
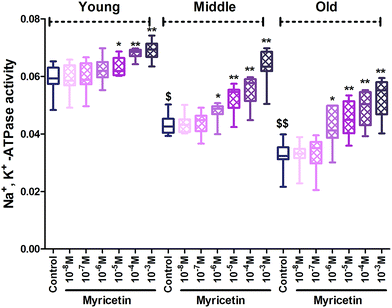
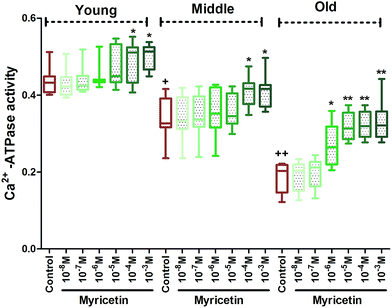
The results of our study showed a significant (p < 0.001) age dependent decline in Na+, K+-ATPase activity in mixed red blood cells population within the groups. In vitro administration of myricetin caused significant (p < 0.05) up-regulation of Na+, K+-ATPase activity at 10−5 M to 10−3 M as compared to their respective controls in all three age group RBCs. Myricetin at 10−6 M showed significant (p < 0.001) up-regulation in enzyme activity only in middle and old age groups as compared to their respective controls. The effect was insignificant at 10−7 M and 10−8 M myricetin in all three age groups as compared to their respective controls (Fig. 2).Fig. 3 showed a significant (p < 0.001) age dependent decrease in Ca2+-ATPase activity in mixed red blood cell population among groups. Co-incubating red blood cells with different concentration (10−8 M to 10−3 M final concentration) of myricetin causes variation in Ca2+-ATPase activity in all three age groups. In vitro treatment of myricetin at 10−3 M and 10−4 M showed significant (p < 0.01) up-regulation of Ca2+-ATPase activity in all three age groups compared to their respective controls but significance (p < 0.001) was more pronounced in old age group. Myricetin at 10−5 M and 10−6 M showed up-regulated activity only in old age group as compared to its control. The effect was insignificant at 10−5 M and 10−8 M myricetin treated red blood cells in young and middle age groups compared to respective control.
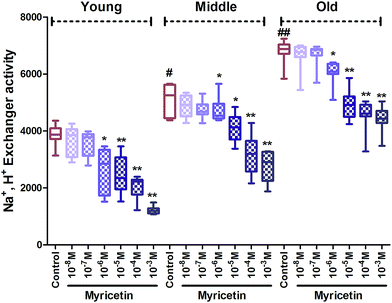
Na+, H+ exchanger activity significantly (p < 0.01) elevated in middle age group as compared to young age group. Significance was even higher (p < 0.001) in the old group as compared to the young control. In vitro treatment of myricetin provides significant fortification against oxidative stress in all age groups. Myricetin caused significant (p < 0.001) inhibition of Na+, H+ exchanger activity compared to respective control at 10−3 M and 10−5 M in all three age group red blood cells. The effect was also significant (p < 0.01) at 10−6 M in young and old age groups but insignificant at 10−7 M and 10−8 M in all three age groups compared to their respective controls (Fig. 4).
A major development over past two decades has been of the realization that free radical mediated oxidative damage is associated with a variety of health problems, such as cancer, neurodegenerative diseases and aging.32 In this study, we propose that myricetin has remarkable antioxidant properties which are evident by up-regulation of Na+, K+-ATPase and Ca2+-ATPase activities, while inhibiting Na+, H+ exchanger activity. Age dependent decline in the activity of ATPases and increase in Na+, H+ exchanger during human aging may be a compensatory response of the individual to an increased oxidative stress.16 Our result shows that myricetin modulates ion transporters in red blood cells. Although the exact mechanisms are not fully understood, however we hypothesize that myricetin acts on ion transporters via indirect mechanisms. The antioxidant scavenging competence of myricetin is connected with the presence of hydroxyl groups (OH) in the B-ring and it may have metal binding sites for ion exchange between the 5-hydroxy and 4-carbonyl group, or between 3′- and 4′-hydroxyl group, thereby chelating metal ions.33 One possible mechanism is the incorporation of myricetin in red blood cell membrane, which may change properties of ion transporters and its activities through non-covalent interactions. It may lead to the change in conformation of ion exchangers. Another possible mechanism is that myricetin may act on phospholipid bilayer, which are easy target of ROS because of unsaturated fatty acids. It has been shown that change in bilayer thickness, fluidity and head group content of membrane affect the activity of ion transporter.34,35 Ion transporter activities depend on properties of cell membrane, and thus inhibition or stimulation by myricetin may cause some changes in physico-chemical properties of the membrane.36 We suggest that the antioxidant action of myricetin alone is not adequate to facilitate cellular aging, but various interactions with distinct intracellular pathways are required. To the best of our knowledge, with this study, we are the first to present concentration dependent effect of myricetin in human red blood cells by detecting Na+, K+-ATPase, Ca2+-ATPase and Na+, H+ exchanger biomarkers during aging. Protective effect of myricetin has been reported against oxidative stress biomarkers which supports our study.37,38 A microarray-based pathway showed that activation of the antioxidant response element is involved in myricetin-induced modulation of gene expression in human hepatoma cells.39 Epidemiological studies suggest that dietary flavonoids ameliorate membrane structure and function against oxidative stress during human aging,40–42 which are in accordance with our results.
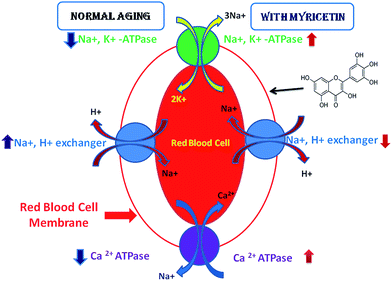
Bioavailability of dietary constituents is a critical mediator of health benefits,9 dose dependent myricetin mediated protection against aging has been studied. We report here that myricetin can ameliorate oxidative stress in red blood cells during human aging. Our findings clearly show that myricetin has potent antioxidant properties that consequently influence cellular aging (Fig. 5).
ROS are one of the products of oxidative reactions which react with polyunsaturated fatty acids (PUFA) of red blood cell membranes, result in lipid peroxidation and alter membrane fluidity.43 ROS can influence the activities of ATPases and Na+, H+ exchanger. We have reported in our previous studies that Na+, K+-ATPase and Ca2+-ATPase activities decrease as a function of human age,16 while Na+, H+ exchanger activity elevates during human aging.17 Na+, K+-ATPase is a dimeric integral membrane enzyme that belongs to P-type ATPases. This ATPase catalyzes the transport of 3Na+ ion outside the cell and 2K+ ion in the cell, there by generating an asymmetric gradient across the plasma membrane.44 A study by Lei et al. demonstrated that myricetin enhances Na+, K+-ATPase activity to protect against D-galactose induced cognitive impairment.45 Ca2+-ATPase also belongs to P-type ATPases which participate as an integral part of the Ca2+ signaling mechanism for eukaryotic cells and crucial component of cell function.46 Na+, H+ exchanger generates a permissive pH level that is critical for the development of mitogenic responses. Also, it has been reported that deletion of Na+, H+ exchanger in mice causes neurological defects, and the growth and viability of those mice was greatly reduced; therefore Na+, H+ exchangers play an important role in cell growth, differentiation and aging.47 Health benefits of myricetin in various diseases have been reported4,48 but little is known about its role in human aging particularly for red blood cells membrane transporters. Authors believe that this study is very novel and it will help in further research on human aging.
Experimental
Materials and methods
Conclusions
Myricetin modulates both ATPases and Na+, H+ exchanger activity during aging in humans. Damage of the plasma membrane occurs directly through interaction with membrane components such as Na+, H+ exchanger, ion dependent ATPase, ion channels and indirectly as a consequence of excess cytosolic damage. Inhibiting function of ion dependent ATPase leads to disturbance in ion homeostasis resulting in impaired signal transduction, altered cellular metabolism, changes in cell membrane permeability and integrity, an elevation in membrane fluidity and disturbances in vital function which finally leads to aging and other age related diseases. Despite this it can be suggested that myricetin may be used as an alternative for available therapeutic strategies for aging and age related disorders. Further research work should be directed towards finding mechanism underlying this effect. These findings emphasize the need to establish age-dependent reference values of dietary flavonoids for oxidative stress biomarkers in different populations and in studies involving their role in different disease conditions.Acknowledgements
PKM and PK acknowledge Amity University Uttar Pradesh, Noida, India for providing necessary research facility and PKM also acknowledge support by fellowship (Science without Borders-Level A) from coordination of Improvement of Higher Education Personnel (CAPES), National Counsel of Technological and Scientific Development (CNPq), Brazil.Notes and references
- J. A. Ross and C. M. Kasum, Annu. Rev. Nutr., 2002, 22, 19–34 CrossRef CAS PubMed.
- D. K. Semwal, R. B. Semwal, S. Combrinck and A. Viljoen, Nutrients, 2016, 8, 90 CrossRef PubMed.
- C. Buchter, D. Ackermann, S. Havermann, S. Honnen, Y. Chovolou, G. Fritz, A. Kampkotter and W. Watjen, Int. J. Mol. Sci., 2013, 14, 11895–11914 CrossRef PubMed.
- M. E. Kassem, L. F. Ibrahim, S. R. Hussein, R. El-Sharawy, M. A. El-Ansari, M. M. Hassanane and H. F. Booles, Pharm. Biol., 2016, 1–6 Search PubMed.
- O. Azevedo Ade, J. J. Campos, G. G. de Souza, C. Veloso Cde, I. D. Duarte, F. C. Braga and C. Perez Ade, J. Nat. Med., 2015, 69, 487–493 CrossRef PubMed.
- C. A. Hobbs, C. Swartz, R. Maronpot, J. Davis, L. Recio, M. Koyanagi and S. M. Hayashi, Food Chem. Toxicol., 2015, 83, 283–292 CrossRef CAS PubMed.
- A. Canuelo, F. J. Esteban and J. Peragon, Eur. J. Nutr., 2016, 55, 639–650 CrossRef CAS PubMed.
- D. J. Aires, G. Rockwell, T. Wang, J. Frontera, J. Wick, W. Wang, M. Tonkovic-Capin, J. Lu, Lezi E, H. Zhu and R. H. Swerdlow, Biochim. Biophys. Acta, 2012, 1822, 522–526 CrossRef CAS PubMed.
- C. Buchter, D. Ackermann, S. Honnen, N. Arnold, S. Havermann, K. Koch and W. Watjen, Food Funct., 2015, 6, 3383–3392 CAS.
- P. K. Maurya, P. Kumar and P. Chandra, World. J. Meth., 2015, 5, 216–222 Search PubMed.
- D. Harman, Biogerontology, 2009, 10, 783 CrossRef PubMed.
- R. S. Franco, Transfus. Med. Hemother., 2012, 39, 302–307 CrossRef PubMed.
- V. Niggli and E. Carafoli, Methods Mol. Biol., 2016, 1377, 57–70 Search PubMed.
- J. Radosinska, L. Mezesova, L. Okruhlicova, K. Frimmel, E. Breierova, M. Bartekova and N. Vrbjar, Clin. Hemorheol. Microcirc., 2016 Search PubMed , epub ahead of print.
- L. Sousa, I. J. Garcia, T. G. Costa, L. N. Silva, C. O. Reno, E. S. Oliveira, C. Q. Tilelli, L. L. Santos, V. F. Cortes, H. L. Santos and L. A. Barbosa, PLoS One, 2015, 10, e0132852 Search PubMed.
- P. K. Maurya and S. Prakash, Appl. Biochem. Biotechnol., 2013, 170, 131–137 CrossRef CAS PubMed.
- P. Kumar, S. Chand and P. K. Maurya, Arch. Physiol. Biochem., 2016, 122, 141–147 CrossRef CAS PubMed.
- R. Whittam and M. E. Ager, Biochem. J., 1965, 97, 214–227 CrossRef CAS PubMed.
- M. F. Pignataro, M. M. Dodes-Traian, F. L. Gonzalez-Flecha, M. Sica, I. C. Mangialavori and J. P. Rossi, J. Biol. Chem., 2015, 290, 6179–6190 CrossRef CAS PubMed.
- B. Bukowska, P. Sicinska, A. Pajak, A. Koceva-Chyla, T. Pietras, A. Pszczolkowska, P. Gorski and M. Koter-Michalak, Biochem. Cell Biol., 2015, 93, 574–580 CrossRef CAS PubMed.
- M. K. Iyer, R. Nayak, R. Colah and S. Chattopadhyay, Free Radical Res., 2013, 47, 710–717 CrossRef CAS PubMed.
- R. Rodrigo, A. Miranda-Merchak, R. Valenzuela Grau, J. P. Bachler and L. Vergara, Clin. Exp. Hypertens., 2014, 36, 17–26 CrossRef CAS PubMed.
- C. Katnik and J. Cuevas, Int. J. Mol. Sci., 2014, 15, 3596–3611 CrossRef CAS PubMed.
- S. Lupachyk, P. Watcho, H. Shevalye, I. Vareniuk, A. Obrosov, I. G. Obrosova and M. A. Yorek, Am. J. Physiol.: Endocrinol. Metab., 2013, 305, E396–E404 CrossRef CAS PubMed.
- K. Srikanthan, J. I. Shapiro and K. Sodhi, Molecules, 2016, 21(9), E1172 CrossRef PubMed.
- S. A. Khan, R. Choudhary, A. Singh and S. H. Bodakhe, J. Curr. Ophthalmol., 2016, 28, 123–130 CrossRef PubMed.
- P. Li, G. R. Chen, F. Wang, P. Xu, L. Y. Liu, Y. L. Yin and S. X. Wang, J. Diabetes Res., 2016, 2016, 1802036 Search PubMed.
- Y. Liu, J. Yang and L. M. Chen, Front. Physiol., 2015, 6, 355 Search PubMed.
- N. E. Hoffman, H. C. Chandramoorthy, S. Shanmughapriya, X. Q. Zhang, S. Vallem, P. J. Doonan, K. Malliankaraman, S. Guo, S. Rajan, J. W. Elrod, W. J. Koch, J. Y. Cheung and M. Madesh, Mol. Biol. Cell, 2014, 25, 936–947 CrossRef PubMed.
- D. G. Le Couteur, S. Solon-Biet, V. C. Cogger, S. J. Mitchell, A. Senior, R. de Cabo, D. Raubenheimer and S. J. Simpson, Cell. Mol. Life Sci., 2016, 73, 1237–1252 CrossRef CAS PubMed.
- D. Ma, Q. Fang, P. Wang, R. Gao, W. Wu, T. Lu, L. Cao, X. Hu and J. Wang, J. Biol. Chem., 2015, 290, 12558–12571 CrossRef CAS PubMed.
- P. K. Maurya, C. Noto, L. B. Rizzo, A. C. Rios, S. O. Nunes, D. S. Barbosa, S. Sethi, M. Zeni, R. B. Mansur, M. Maes and E. Brietzke, Prog. Neuro-Psychopharmacol. Biol. Psychiatry, 2016, 65, 134–144 CrossRef CAS PubMed.
- Y. Guo and R. S. Bruno, J. Nutr. Biochem., 2015, 26, 201–210 CrossRef CAS PubMed.
- M. Gustavsson, N. J. Traaseth and G. Veglia, Biochemistry, 2011, 50, 10367–10374 CrossRef CAS PubMed.
- J. Li, Z. M. James, X. Dong, C. B. Karim and D. D. Thomas, J. Mol. Biol., 2012, 418, 379–389 CrossRef CAS PubMed.
- D. Blaskovic, P. Zizkova, F. Drzik, J. Viskupicova, M. Veverka and L. Horakova, Interdiscip. Toxicol., 2013, 6, 3–8 Search PubMed.
- K. B. Pandey, N. Mishra and S. I. Rizvi, Nat. Prod. Commun., 2009, 4, 221–226 CAS.
- Y. Song, J. E. Manson, J. E. Buring, H. D. Sesso and S. Liu, J. Am. Coll. Nutr., 2005, 24, 376–384 CrossRef CAS PubMed.
- S. Qin, J. Chen, S. Tanigawa and D. X. Hou, Mol. Nutr. Food Res., 2013, 57, 435–446 CAS.
- P. Kumar and P. K. Maurya, Adv. Pharm. Bull., 2014, 4, 443–447 CAS.
- P. Kumar and P. K. Maurya, Rejuvenation Res., 2013, 16, 179–184 CrossRef CAS PubMed.
- S. D'Angelo, F. Trojsi, A. Salvatore, L. Daniele, M. Raimo, P. Galletti and M. R. Monsurro, Neurochem. Int., 2013, 63, 626–634 CrossRef PubMed.
- F. A. Ansari and R. Mahmood, J. Agric. Food Chem., 2015, 63, 10372–10379 CrossRef CAS PubMed.
- M. G. Palmgren and P. Nissen, Annu. Rev. Biophys., 2011, 40, 243–266 CrossRef CAS PubMed.
- Y. Lei, J. Chen, W. Zhang, W. Fu, G. Wu, H. Wei, Q. Wang and J. Ruan, Food Chem., 2012, 135, 2702–2707 CrossRef CAS PubMed.
- E. E. Strehler, A. J. Caride, A. G. Filoteo, Y. Xiong, J. T. Penniston and A. Enyedi, Ann. N. Y. Acad. Sci., 2007, 1099, 226–236 CrossRef CAS PubMed.
- Z. Lu, L. Yao, Z. Jiang, J. R. Aschenbach, H. Martens and Z. Shen, J. Dairy Sci., 2016, 99, 733–745 CrossRef CAS PubMed.
- H. M. Su, L. N. Feng, X. D. Zheng and W. Chen, J. Zhejiang Univ., Sci., B, 2016, 17, 437–446 CrossRef CAS PubMed.
- P. K. Maurya, P. Kumar and P. Chandra, Arch. Physiol. Biochem., 2016, 122, 61–66 CrossRef CAS PubMed.
- V. T. Marchesi and G. E. Palade, J. Cell Biol., 1967, 35, 385–404 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- C. H. Fiske and Y. Subbarow, Science, 1927, 65, 401–403 CAS.
- T. S. Teo, P. Thiyagarajah, Z. Z. Wong and N. P. Das, Biochem. Med. Metab. Biol., 1991, 45, 209–215 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |