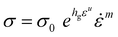Diglycidylether of iso-eugenol: a suitable lignin-derived synthon for epoxy thermoset applications
Camille Françoisa,
Sylvie Pourcheta,
Gilles Bonia,
Stéphane Fontaineb,
Yves Gaillardc,
Vincent Placetc,
Maxim V. Galkind,
Alexander Orebomd,
Joseph Samec*d and
Laurent Plasseraud*a
aICMUB Institute, Department of Electrochemistry, Molecular Materials and Devices, UMR-CNRS 6302, University of Bourgogne Franche-Comté, 9 avenue Alain Savary, F-21078 Dijon, France. E-mail: Laurent.plasseraud@u-bourgogne.fr; Tel: +33 3 80 39 91 60
bDRIVE Laboratory, Institut Supérieur de l'Automobile et des Transports, 49 rue Melle Bourgeois, F-58027 Nevers, France
cFEMTO Institute, Applied Mechanics Department, UMR CNRS 6174, University of Franche-Comté, 24 Chemin de l'Epitaphe, F-25000 Besançon, France
dDepartment of Organic Chemistry, Arrhenius Laboratory, Stockholm University, S-106 91 Stockholm, Sweden. E-mail: joseph.samec@kemi.uu.se
First published on 14th July 2016
Abstract
A novel lignin-based synthon, diglycidylether of iso-eugenol (DGE-isoEu) is used as a prepolymer for the preparation of thermosetting resins. DGE-isoEu is synthesized in a two-step procedure with a satisfactory yield from bio-based iso-eugenol (isoEu, 2-methoxy-4-(1-propenyl)phenol) catalytically fragmented from lignin in an organosolv process. DGE-isoEu was fully characterized by NMR, MS and FTIR. Curing of the DGE-isoEu monomer has then been investigated in the presence of several carboxylic acid derivatives hardeners. The thermal and mechanical properties of each material were recorded showing, in particular, a high Tg and instantaneous modulus values in the range of 78–120 °C and 4.6–5.5 GPa, respectively. The lignin derived new materials give very attractive thermo-mechanical properties comparable to that of common BPA-containing epoxy resins.
1. Introduction
Until the 2000s, bisphenol-A (BPA) played a significant role in the field of polymers and was widely used as a basic building block for the preparation of polycarbonates, polysulfones, polyesters, polyimides, and epoxy resins.1 Thereafter, toxicological studies have suspected a potential risk for health in the use of BPA-based materials, which was later classified as an endocrine-disrupting chemical.2,3 As many applications involving BPA concern the areas of food and health, the quest of non-toxic alternatives to BPA has become a major focus and has attracted great interest among the communities of chemists and polymerists.4 Thus, in the sector of epoxy resins, the replacement of a diglycidylether of bisphenol A (DGEBA) by bio-based synthons is strongly considered.5 Beyond the toxicological aspects, and from an environmental and energy perspective, this involves the replacement of petroleum-based building-blocks by monomers from renewable resources.During the last decade, advances in the catalytic conversion of biomass have led to a wide range of useful platform molecules.6 This approach is also widespread in the field of polymers.7,8 In the specific domain of biobased epoxy thermosets, the state of art was recently and concomitantly reviewed by David et al., and by Matharu and Ding, highlighting the potential of polyphenols, tannins, cardanol, vegetable oils, starch, sugar, and terpenes employed as precursors for the synthesis of epoxy monomers.9,10 Aromatic molecules extracted during the fragmentation of the biomass, in particular from the lignin, can be also considered as appropriate candidates.11–13 Indeed, the thermomechanical properties recorded for some of these building blocks (revealed by high Tg values), and mainly derived from vanillin, are very competitive to DGEBA-based thermosets.14–16 However, with the objective of future industrial developments, accessibility and availability of raw materials are key criteria. The lignin which is produced in abundant amounts (an estimated 50 million tonnes of lignin are annually produced worldwide by the pulp and paper industry) well fits with these conditions and constitutes a remarkable pool of high-added value aromatic molecules.17 In this context, the Samec's research group (Stockholm) recently reported a tandem organosolv and palladium-catalyzed depolymerisation process to fractionate lignocellulosic biomass into cellulose, hemicellulose and an iso-eugenol rich of ligninoil.18 This innovative process provides access to bio-based iso-eugenol which is a precursor for the synthesis of vanillin.19
In our ongoing studies on the chemical modification of biobased building-blocks for material applications,20,21 we herein report the chemical transformation of iso-eugenol isolated from lignin into a diglycidylether derivative (DGE-isoEu) and its use as a prepolymer in the preparation of epoxy thermosets together with several acid derivatives hardeners. Although amines are the most used hardeners, this family of curing agents was preferentially selected because they also can be efficiently prepared from biorenewable resources.22 DGE-isoEu was fully characterized by nuclear magnetic resonance (NMR), mass spectrometry (MS) and Fourier transform infrared spectroscopy (FT-IR). The polymerization and the resulting materials were monitored and analysed by differential scanning calorimetry (DSC), thermogravimetric analyses (TGA), and nanoindentation. Finally, the curing properties of DGE-isoEu, directly derived from lignin, are compared to those of diglycidylether of eugenol (DGE-Eu), derived from clove, and recently reported by Zhang et al.23
2. Materials and methods
2.1. Materials
2.2. Methods
1H NMR (400 MHz, CDCl3) δ 6.87 (m, 1H, Ar), 6.81 (m, 1H, Ar), 6.73 (m, 1H, Ar), 4.21 (dd, J = 11.4 and 3.5 Hz, 1H, CH2O), 4.00 (dd, J = 11.4 and 5.6 Hz, 1H, CH2O), 3.84 (s, 3H, Me), 3.51 (d, J = 2.1 Hz, 1H, CH(O)CHMe), 3.35 (m, 1H), 2.99 (qd, J = 5.1 and 2.1 Hz, 1H, CH(O)CHMe), 2.86 (dd, J = 4.9 and 4.2 Hz, 1H), 2.70 (dd, J = 4.9 and 2.7 Hz, 1H), 1.41 (d, J = 5.1 Hz, 3H, Me); cis isomer (incomplete): 3.85 (s, 3H, Me), 3.28 (m, 1H), 1.07 (d, J = 5.5 Hz, 3H, Me); 13C{1H} NMR (100 MHz, CDCl3) δ 149.8, 147.8, 131.4, 118.2, 114.0, 108.6, 70.3, 59.4, 58.8, 55.8, 50.1, 44.8, 17.7; MS (EI) (m/z) (rel. intensity): 236 (M+, 45%), 207 (18%), 193 (55%), 151 (14%), 137 (100%), 122 (10%), 107 (8%), 91 (10%), 77 (10%); IR (neat, cm−1): νmax 3000, 2964, 2930, 1609, 1593, 1515, 1461, 1417, 1378, 1352, 1319, 1269, 1247, 1229, 1195, 1162, 1137, 1081, 1022, 971, 928, 912, 862, 840, 803, 757, 720.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 molar ratio were first ground in a mortar at room temperature until an homogeneous mixture is obtained. EMID used as catalyst ratio 0.08 eq. or 0.12 eq. with respect to curing agent (see details in Table 1) was then added and intimately ground. The 1
0.8 molar ratio were first ground in a mortar at room temperature until an homogeneous mixture is obtained. EMID used as catalyst ratio 0.08 eq. or 0.12 eq. with respect to curing agent (see details in Table 1) was then added and intimately ground. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 molar ratio instead of a 1
0.8 molar ratio instead of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used to take into account the possible reaction involving hydroxyl groups formed and epoxy functions, leading to polyether.
1 was used to take into account the possible reaction involving hydroxyl groups formed and epoxy functions, leading to polyether.
| Hardeners | Molar ratio (DGE-isoEu/curing agent/catalyst) |
|---|---|
| MPA | 1/0.8/0.12 |
| HHPA | 1/0.8/0.08 |
| THPA | 1/0.8/0.08 |
| PA | 1/0.8/0.08 |
| MA | 1/0.8/0.08 |
| FDCA | 1/0.8/0.08 |
A few milligrams of this homogeneous mixture was placed in a DSC pan in order to study polymerisation reaction. The remaining mixture was transferred in an aluminium pan and cured in an oven at 150 °C during 1 h.
2.3. Characterization techniques
Finally, nanoindentations at constant ḣ/h have been also performed in order to determine the strain rate sensitivity (m). Assuming that the material follows a G'Sell–Jonas law:24
![[small epsi, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a1.gif) is the strain rate and hg and u are parameters characteristic of strain hardening. The strain rate sensitivity can be calculated as follow:
is the strain rate and hg and u are parameters characteristic of strain hardening. The strain rate sensitivity can be calculated as follow:where F|h is the applied force at a constant penetration depth (here 150 nm). ḣ/h between 0.001 s−1 and 0.5 s−1 have been applied. More details can be found in Gaillard et al.25
3. Results and discussion
3.1. Synthesis of DGE-isoEu
The diglycidylether was prepared from iso-eugenol (isoEu) in two reaction steps (Scheme 2). IsoEu was treated with epichlorohydrin, in the presence of a mild base to generate the corresponding mono-epoxy iso-eugenol. It should be noted that epichlorohydrin can be generated from glycerol.26 This species was then epoxidized using oxone or alternatively using hydrogen peroxide (tungsten-catalyzed) to generate the product in a 92% yield. DGE-isoEu constitutes an original synthon derived from the lignin. Fully characterized (see Section 2.2.1), its 1H NMR spectrum is depicted in Fig. 2b and compared to that of the isoEu precursor (Fig. 2a).3.2. Epoxy thermosets syntheses
Epoxy thermosets were synthesized by heating mixtures of epoxy prepolymer, acid derivatives curing agents and 2-ethyl-4-methylimidazole (EMID) used as a suitable catalyst.27 This study mainly concerns anhydride acid hardeners and was also extended to the bio-sourced 2,5-furandicarboxylic acid.28 The anionic polymerization occurs according a ring-opening mechanism catalysed by EMID. In the past, this mechanism has been studied and described first by Dusek and then by Montero.29,30 Depending on the nature of both resin (DGE-Eu or DGE-isoEu) and hardener, the resulting mixture could either be in a powder form or in the form of a waxy paste more or less viscous at room temperature. This obviously makes the mixing operation more or less easy. This practical aspect should be taken in consideration for a potential composite application. The facility of mixing and the consistency of the different formulations at room temperature are reported in Table 2. Two DGE-Eu-based mixtures were prepared and inserted for comparison.| Reaction mixture | Physical aspect of the mixture at room temperature | Facility of mixing |
|---|---|---|
a  |
||
| DGE-Eua/MPA | Cohesive powder | Uneasy |
| DGE-Eua/HHPA | Waxy paste | Easy |
| DGE-isoEu/MPA | Cohesive powder/waxy paste | Uneasy |
| DGE-isoEu/HHPA | Waxy paste | Easy |
| DGE-isoEu/THPA | Waxy paste | Uneasy |
| DGE-isoEu/PA | Waxy paste | Uneasy |
| DGE-isoEu/MA | Liquid – strong viscosity increasing due to rapid polymerization | Uneasy (polymerization) |
| DGE-isoEu/FDCA | Powder | Uneasy |
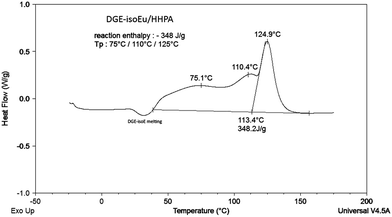
It should be noted that the formulation DGE-isoEu/MA gives rise to a fast and uncontrolled polymerization as soon as the mixture is ground at room temperature leading to a viscosity increase of the paste. Immediately after mixing and for each formulation, curing reaction was followed by differential scanning calorimetry by heating the sample from −20 °C to 180 °C at a heating rate of 20 °C min−1. In accordance with Zhang et al. exothermic peaks related to the polymerization reaction are observed between 50 °C and 152 °C depending on the formulation (see Table 3).23 These multiple peaks are probably due to different curing reactions occurring between epoxy and anhydride/acid functions. Indeed each DGE-Eu or DGE-isoEu contains two epoxy functions having different reactivities. It is worth noting that a previous endothermic melting peak of DGE-isoEu is already observed at 45 °C when this resin is used (see Fig. 3).
| Thermosets | Tg (°C) | T polymerization (°C) | ΔH (kJ mol−1 epoxy) | ||
|---|---|---|---|---|---|
| a Ref. 23. | |||||
| DGE-Eu/MPAa | 155 | 106 | 127 | 139 | 73 |
| DGE-Eu/HHPAa | 114 | 119 | 130 | 80 | |
| DGE-isoEu/MPA | 93 | 53 | 130 | 51 | |
| DGE-isoEu/HHPA | 120 | 75 | 110 | 125 | 63 |
| DGE-isoEu/THPA | 107 | 95 | 123 | 70 | |
| DGE-isoEu/PA | 120 | 66 | 110 | 132 | 54 |
| DGE-isoEu/MA | 93 | 58 | 120 | 135 | 36 |
| DGE-isoEu/FDCA | 78 | 53 | 123 | 55 | |
Reaction enthalpy was also determined for each polymerization reaction and these values were then expressed by mole of epoxy function in order to take into account the different hardeners molar masses. These data are reported in Table 3. Polymerization enthalpies recorded are significantly lower when DGE-isoEu is used instead of DGE-Eu. This could be due to the lower reactivity of the internal epoxy function of the DGE-isoEu. However it is worth noting that polymerization enthalpy measured results from both the value of the reaction enthalpy and the progress of the reaction. Unfortunately the values of the reaction enthalpies related to the polymerization of hardener (HHPA/MPA) with DGE-Eu or DGE-isoEu are not known and could differ according to the position of the epoxy function. Consequently it seems difficult to compare the epoxy reactivity between DGE-isoEu and DGE-Eu only from the polymerization enthalpies measured.
Polymerization enthalpies are in the range of 50 to 80 kJ mol−1 except for MA. For MA, as the polymerization reaction begins before the DSC analysis, a low value of 36 kJ mol−1 is recorded. Concerning the MPA hardener the same trend was already reported with DGE-Eu.23 This is partly due to low function (anhydride/acid) density of MPA in connection with its high molar mass. Furthermore in this case the anhydride function carried by an hindered molecule could be less reactive with the internal epoxy group of DGE-isoEu than with the terminal epoxy function of DGE-Eu.
3.3. Characterization of epoxy thermosets
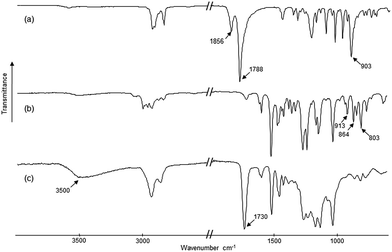
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester stretching band at 1730 cm−1. At the same time the epoxy bands in the range of 915–800 cm−1, present in DGE-isoEu spectra, and the anhydride elongation bands at 1856, 1788 cm−1 (C
O ester stretching band at 1730 cm−1. At the same time the epoxy bands in the range of 915–800 cm−1, present in DGE-isoEu spectra, and the anhydride elongation bands at 1856, 1788 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and at 903 cm−1 (C–O–C cyclic anhydride), present in HHPA spectra (a), have disappeared. Concomitantly, according to the proposed mechanism in the presence of labile proton,14 an O–H elongation band at about 3500 cm−1 resulting from the opening of the epoxy ring appears in the polymer IR spectrum. Comparable IR fingerprints were recorded for the other mixtures reported in Table 1.
O) and at 903 cm−1 (C–O–C cyclic anhydride), present in HHPA spectra (a), have disappeared. Concomitantly, according to the proposed mechanism in the presence of labile proton,14 an O–H elongation band at about 3500 cm−1 resulting from the opening of the epoxy ring appears in the polymer IR spectrum. Comparable IR fingerprints were recorded for the other mixtures reported in Table 1.
| Prepolymer | DGE-isoEu | |||||
|---|---|---|---|---|---|---|
| Curing agent | MA | PA | HHPA | THPA | MPA | |
| Nano-indentation | ER (GPa) average standard deviation | 4.86 | 4.47 | 4.15 | 4.59 | 4.52 |
| 0.04 | 0.02 | 0.03 | 0.09 | 0.22 | ||
| EI (GPa) average standard deviation | 5.54 | 4.71 | 4.60 | 5.18 | 5.29 | |
| 0.10 | 0.16 | 0.08 | 0.15 | 0.87 | ||
| H (MPa) average standard deviation | 395 | 345 | 338 | 342 | 229 | |
| 6.5 | 34.0 | 7.2 | 10.0 | 83.0 | ||
| m (Ø) average standard deviation | 0.065 | 0.025 | 0.026 | 0.028 | 0.001 | |
| 0.027 | 0.008 | 0.007 | 0.011 | 0.047 | ||
As expected, values of instantaneous modulus are larger than the relaxed ones. They are ranged from 4.15 GPa to 5.54 GPa. Hardness (H) vary between 230 MPa and 400 MPa. These properties are of the same order of magnitude than common petroleum-based resins. The Table 5 shows also the value obtained for the strain rate sensitivity (m). They are in good accordance with typical values encountered for classical polymer materials.31 It has been impossible to prepare the surface of the FDCA sample for such nanoindentation experiments.
4. Conclusions
Diglycidylether of iso-eugenol was successfully produced from iso-eugenol, with a very good yield, according to a two-step process, and then used as a thermosetting epoxy prepolymer. The study of the polymerization conducted in the presence of several acid derivatives based hardeners has highlighted satisfactory thermo-mechanical properties tailored for such material applications. Indeed, and based on their high Tg, elastic modulus E, and hardness values recorded in the presence of HHPA, THPA and PA hardeners, these new bio-based thermoset polymers show promising mechanical properties. Nanoindentation measurements have also shown that these materials have a good homogeneity. Thus, the diglycidylether of iso-eugenol can be considered as a suitable new epoxy prepolymer. Moreover, as iso-eugenol is directly prepared from lignin fragmentation by a catalytic process, the availability of diglycidylether of iso-eugenol is greatly facilitated, which enhances its attractiveness as precursor of biosourced epoxy resin. Further work is currently underway to achieve fully bio-based epoxy thermosets and to evaluate the potential of these resins for the manufacturing of composites.Acknowledgements
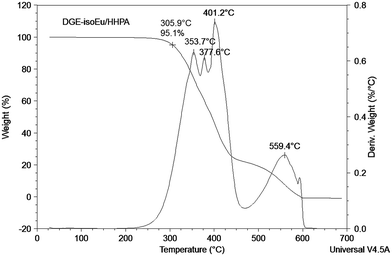
The authors would like to acknowledge Julien Guerin for the TGA analysis. The authors are grateful for general and financial support from the Centre National de la Recherche Scientifique (CNRS-France), the University of Bourgogne Franche-Comté (BQR PRES 2014–2016 Bourgogne Franche-Comté) and the Institut Français in Sweden (TOR 2015 mobility program). C. F. is thankful for a PhD fellowship awarded by the Conseil Régional de Bourgogne (France) in the frame of the “Jeunes Chercheurs Entrepreneurs-2016” program and in partnership with AgroComposites Entreprises.References
- T. Geens, L. Goeyens and A. Covaci, Int. J. Hyg. Environ. Health, 2011, 214, 339–347 CrossRef CAS PubMed.
- P. Fenichel, N. Chevalier and F. Brucker-Davis, Annee Endocrinol., 2013, 74, 211–220 CrossRef CAS PubMed.
- J. Michałowicz, Environ. Toxicol. Pharmacol., 2014, 37, 738–758 CrossRef PubMed.
- A. M. Nelson and T. E. Long, Polym. Int., 2012, 61, 1485–1491 CrossRef CAS.
- A. Maiorana, S. Spinella and R. A. Gross, Biomacromolecules, 2015, 16, 1021–1031 CrossRef CAS PubMed.
- M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827–1870 CrossRef CAS PubMed.
- M. A. R. Meier, J. O. Metzger and U. S. Schubert, Chem. Soc. Rev., 2007, 36, 1788–1802 RSC.
- A. Gandini, T. M. Lacerda, A. J. F. Carvalho and E. Trovatti, Chem. Rev., 2016, 116, 1637–1669 CrossRef CAS PubMed.
- R. Auvergne, S. Caillol, G. David, B. Boutevin and J.-P. Pascault, Chem. Rev., 2014, 114, 1082–1115 CrossRef CAS PubMed.
- C. Ding and A. S. Matharu, ACS Sustainable Chem. Eng., 2014, 2, 2217–2236 CrossRef CAS.
- P. Feng and F. Chen, BioResources, 2012, 7, 2860–2870 CrossRef.
- T. Koike, Polym. Eng. Sci., 2012, 52, 701–717 CAS.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, Macromol. Rapid Commun., 2016, 37, 9–28 CrossRef CAS PubMed.
- M. Fache, E. Darroman, V. Besse, R. Auvergne, S. Caillol and B. Boutevin, Green Chem., 2014, 16, 1987–1998 RSC.
- M. Fache, B. Boutevin and S. Caillol, Eur. Polym. J., 2015, 68, 488–502 CrossRef CAS.
- M. Fache, A. Viola, R. Auvergne, B. Boutevin and S. Caillol, Eur. Polym. J., 2015, 68, 526–535 CrossRef CAS.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Science, 2014, 344, 709–718 CrossRef CAS PubMed.
- M. V. Galkin and J. S. M. Samec, ChemSusChem, 2014, 7, 2154–2158 CrossRef CAS PubMed.
- P. Pfeifer, A. Zurawski and T. Roeder, Naturwiss. Unterr. Chem., 2004, 15, 34–37 CAS.
- A. Mhanna, F. Sadaka, G. Boni, C.-H. Brachais, L. Brachais, J.-P. Couvercelle, L. Plasseraud and L. Lecamp, J. Am. Oil Chem. Soc., 2014, 91, 337–348 CrossRef CAS.
- S. Bigot, M. Daghrir, A. Mhanna, G. Boni, S. Pourchet, L. Lecamp and L. Plasseraud, Eur. Polym. J., 2016, 74, 26–37 CrossRef CAS.
- C. Robert, F. de Montigny and C. M. Thomas, ACS Catal., 2014, 4, 3586–3589 CrossRef CAS.
- J. Qin, H. Liu, P. Zhang, M. Wolcott and J. Zhang, Polym. Int., 2014, 63, 760–765 CrossRef CAS.
- C. G'Sell and J. Jonas, J. Mater. Sci., 1979, 14, 583–591 Search PubMed.
- Y. Gaillard, M. Girard, G. Monge, A. Burr, E. Darque Ceretti and E. Felder, J. Appl. Polym. Sci., 2013, 128(5), 2713–2719 CrossRef CAS.
- B. M. Bell, J. R. Briggs, R. M. Campbell, S. M. Chambers, P. D. Gaarenstroom, J. G. Hippler, B. D. Hook, K. Kearns, J. M. Kenney, W. J. Kruper, D. J. Schreck, C. N. Theriault and C. P. Wolfe, Clean, 2008, 36, 657–661 CAS.
- K. Huang, Z. Liu, J. Zhang, S. Li, M. Li, J. Xia and Y. Zhou, Eur. Polym. J., 2015, 70, 45–54 CrossRef CAS.
- J. Zhang, J. Li, Y. Tang, L. Lin and M. Long, Carbohydr. Polym., 2015, 130, 420–428 CrossRef CAS PubMed.
- K. Dusek, Adv. Polym. Sci., 1986, 78, 1–59 CrossRef CAS.
- B. Montero, A. Serra, C. Ramirez and X. Ramis, Polym. Compos., 2013, 34, 96–108 CrossRef CAS; J. L. Bucaille, E. Felder and G. Hochstetter, J. Mater. Sci., 2002, 37, 3999–4011 CrossRef.
- H. Lee and K. Neville, Handbook of Epoxy Resins, McGraw-Hill, New York 1967 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |