Synthesis and optical characterization of a high-quality ZnS substrate for optoelectronics and UV solar-energy conversion†
Ching-Hwa Ho *ab and
Min-Han Lina
*ab and
Min-Han Lina
aGraduate Institute of Applied Science and Technology, National Taiwan University of Science and Technology, Taipei 106, Taiwan. E-mail: chho@mail.ntust.edu.tw; Fax: +886 2 27303733; Tel: +886 2 27303772
bGraduate Institute of Electro-Optical Engineering and Department of Electronic and Computer Engineering, National Taiwan University of Science and Technology, Taipei 106, Taiwan
First published on 12th August 2016
Abstract
ZnS is an environmentally friendly wide-band-gap semiconductor possessing a direct gap larger than ZnO, which makes it more promising for application in ultraviolet (UV) optoelectronics and solar-energy conversion applications. However, highly crystalline ZnS is usually obtained by elegant epitaxial growth, such as with pulsed laser vaporization, molecular beam epitaxy, and metal–organic chemical vapour deposition, in a thin-film form. A high-quality ZnS substrate crystal has been rarely achieved to date. Herein, we demonstrate a high-grade cubic ZnS (c-ZnS) substrate crystal with a longer range order grown by a chemical vapour transport (CVT) method. Photoluminescence (PL), transmittance, contactless electroreflectance (CER), and transmission electron microscopy were performed for qualification of the c-ZnS substrate. The transparency of the c-ZnS substrate crystal (∼0.6 mm thick) was about 81% at λ = 400 nm and 92% at λ = 600 nm. Strong and complete series excitonic emissions were detected from the ZnS substrate crystal by PL, indicating the high crystallinity of the CVT-grown ZnS. Band-edge free-exciton emission of the ZnS substrate showed an intense emission around 3.68 eV at 300 K. The temperature dependences of the band-edge excitonic emissions from 10 to 300 K were analyzed and are herein discussed in terms of sustaining the high crystalline quality of the c-ZnS substrate. An initially-formed Cu/ZnS Schottky solar cell was also tested using the c-ZnS substrate. Under the illumination of a 325 nm laser (i.e. a power density of P ∼ 48 mW cm−2), a significant photovoltage of ∼0.6 V could be generated from the original Cu/ZnS Schottky solar cell.
1. Introduction
Recently, semiconductor optoelectronic technology has developed rapidly alongside the recent advances in the epitaxial-growth technique for achieving various device structures in micro/nanoelectronics, optoelectronics and photovoltaic applications. There are two main elements of epitaxial growth essential to form a device structure: the “substrate” and the “epilayer”. High-quality epilayers need to be based on an excellent substrate with high crystallinity to achieve epitaxial growth.As one of the most important II–VI group semiconductors, zinc sulfide (ZnS) is a direct semiconductor with a band gap (∼3.7 eV)4–6 larger than that of ZnO (∼3.3 eV),7,8 which makes it more suitable and flexible for application in ultraviolet (UV) to visible optoelectronics devices. ZnS has generally two crystalline phases: cubic (sphalerite) and hexagonal (wurtzite).9 The wurtzite ZnS possesses an energy gap (∼3.8 eV) slightly larger than that of cubic ZnS (c-ZnS).10 Despite this, the crystal planes of the wurtzite {0001} and zinc blende (cubic) {111} forms of ZnS have been claimed to possess the same atomic arrangement and hence comparable energy states of the crystal,11 therefore one could postulate that c-ZnS might have the optoelectronics properties of both wurtzite and sphalerite along different crystal planes. High-quality ZnS thin films can be obtained by growing the epitaxial layers and nanostructures on various substrates, such as quartz,12 Si,1,13,14 GaAs,3,15,16 GaP2,17 and other plates with a sufficient high crystal quality to suit the possible luminescence device applications. Zinc sulfide easily forms structural defects in the crystal, such as sulfur vacancies, twins and stacking faults.1,18 This is the reason that low-dimensional and small-size crystallites, such as nanowires, nanobelts and thin-film type ZnS, can readily achieve a highly crystalline quality. Large size and longer-range-order ZnS substrate crystals are relatively much harder to be grown with respect to the other thin-film type growth. In particular, using ZnS as the substrate for the epitaxy will directly lead to the formation of a direct-gap heterojunction, which contributes to the carriers and optoelectronics of the devices. The specific functioning of the ZnS is different to the other substrates of quartz, sapphire and Si.
The UV band-edge emission can be a qualification index for evaluation of the highly crystalline quality of the as-grown ZnS. Despite various device applications of ZnS, there are still no reports in the literature of high-quality inorganic ZnS “substrate crystal” with a complete series of band-edge excitonic emissions. In this paper, we report our study to grow high-quality ZnS bulk crystals with a size larger than 1 cm by a chemical vapour transport (CVT) method with I2 as the transport agent. X-ray diffraction (XRD) and high-resolution transmission electron microscopy (HRTEM) were used to verify the crystalline phase (i.e. c-ZnS) and crystallinity of the as-grown zinc sulfide. An initial cutting and polishing of the c-ZnS substrate crystal allowed us to hand-make a crystal with a size of 5 × 3 × 0.6 mm3, which showed a visible-light transparency of ∼81% at 400 nm and ∼92% at 600 nm for the ZnS substrate. At 300 K, a strong band-edge emission of ∼3.68 eV was emitted from the c-ZnS substrate in the photoluminescence (PL) measurements, which matched well with the interband transition of band-edge free exciton (FX) measured by the transmittance (T) and contactless electroreflectance (CER) analyses. Complete excitonic-emission features including FX, bi-excitons (XX), neutral donor-bound excitons (DX0), neutral acceptor-bound excitons (AX0) and longitudinal optical (LO) phonon replica were clearly detected near the band edge of the c-ZnS substrate at low temperature, indicating the superior crystal quality of the zinc sulfide substrate with a larger size and longer range order. An initially-formed Cu/c-ZnS Schottky solar cell was also made and tested with the c-ZnS substrate crystal. A peak photovoltaic responsivity of ∼0.14 V mW−1 (i.e. an optical spot size of ∼100 μm) was obtained near 3.722 eV in the spectral response of the simple Schottky solar cell. This result indicates that the c-ZnS substrate is appropriate for UV solar energy conversion, especially for the outer-space (universe) use for electricity production.
2. Experimental section
Bulk single crystals of ZnS were grown by the CVT method with iodine as the transport agent. The CVT growth was performed in a horizontal three-zone tube furnace with a temperature gradient setting as 950 °C ← 1000 °C → 950 °C for simultaneously growing two sealed quartz ampoules (22 mm OD and 17 mm ID, 20 cm length).19 The temperature gradient was about −2.5 °C cm−1. Prior to the crystal growth, the pure elements of Zn (99.99% pure) and S (99.999% pure) powder in a stoichiometric amount together with small amount of transport agent (I2, ∼10 mg cm−3) were put into the quartz ampoules, which were then cooled with liquid nitrogen, evacuated to ∼10−6 Torr and sealed. The growth kinetics of CVT with the auxiliary use of a transport agent can be described as iA(s) + jB(g) ⇆ kC(g), where A is the synthesized material, B is the transport agent and C is the gaseous synthesized compound (A, C = ZnS and B = I2). The formation of ZnS involves the reverse reaction of the above equation. In general, the use of a transport agent is to facilitate the gaseous transport of ZnS from high temperature (1000 °C) to low temperature (950 °C) inside the quartz ampoule. This reaction may take up to 240 h to grow large single crystals. The optical measurements of CER and transmittance were implemented in a 0.2 m PTI monochromatic system, where a 150 W xenon arc lamp acted as the white light source.7 A 1200 groves per mm ruled grating acted as the dispersion unit of the white light. The measurement range of the CER and transmittance was from 2.5 to 4.5 eV. The PL measurements were implemented in a spectral measurement system in which an iHR 550 imaging spectrometer equipped with a 2400 groves per mm grating acted as the dispersion unit. The CCD array detection was employed in the PL measurement. The pumping light source was a Q-switched diode-pumped solid-state laser of λ = 266 nm and the spot size was reduced to ∼100 μm. A set of neutral density filters changed and controlled the pumping power of the laser. For the low-temperature and temperature-dependent PL measurements, a He-compressed RMC 22 closed-cycle refrigerator equipped with a thermometer controller facilitated the experiments. For the photovoltaic-related measurements of the Cu/ZnS Schottky solar cell, an initially-formed Schottky solar cell was made by first attaching the ZnS substrate crystal onto a copper sample holder using silver paste, and then contacting this with the Cu mesh directly onto the top surface of the ZnS. The same monochromatic system as that used for the CER and transmittance measurements was used. The photoexcited electron–hole pairs from the surface band-bending region were extracted out from the top and bottom electrodes of the capacitor-like configuration, and then connected to a low-noise preamplifier. The measured photovoltaic responsivity of the Schottky solar cell was calibrated via an OPHIR optical power meter equipped with broadband and a high sensitivity thermal sensor suitable for the wavelength range 0.15–6 μm.3. Results and discussion
Fig. 1a shows the as-grown crystals of bulk ZnS by the CVT method. The crystals essentially display as white or transparent, with some of them showing a little bit as light-yellow coloured, with a crystal size up to ∼1 cm. The characteristic transparent and clear colour of the as-grown ZnS indicates a lower defect and impurity density and suggests the wide-band-gap characteristic of the zinc sulfide crystals. The lower-right inset in Fig. 1a also reveals the bright shiny surface of the as-grown ZnS grown by CVT. To determine the crystalline phase, some smaller crystals of the as-grown ZnS were finely ground into powder and then a powder XRD pattern was taken, as shown in Fig. 1b. The XRD pattern matched well with the JCPDF no. 772100 for a cubic (sphalerite) ZnS with a zinc blende structure, as shown in Fig. 1b. Three enhanced peaks of (111) at ∼28.32°, (220) at ∼47.27° and (311) at ∼56.15° with the intensity order I(111) > I(220) > I(311) identified the zinc blende phase of the as-grown ZnS (i.e. c-ZnS).20 The inset in Fig. 1b shows the full-width half maximum (FWHM) of the most prominent (111) peak by line-shape fit, which is about 0.1°, thus verifying the good crystalline quality of the c-ZnS. The higher-order peaks, such as (331) near 76.5°, reveal two features. They are originated from an X-ray line simultaneously consisting of Cu Kα1 and Cu Kα2 emissions. Besides, a bulk XRD pattern of the crystal was also taken and showed only one (220) peak of the cubic ZnS. The sphalerite ZnS of the zinc blende structure is depicted as the representative scheme in Fig. 1c. Each cubic unit cell consists of four sp3 pyramids centred by one Zn atom, which is connected to the surrounding four nearest S atoms. The fundamental sp3 pyramid (connected with four Z–S bonds of the angle of ∼109°) may comprise the main band structure of the c-ZnS; we measure and discuss the main band-edge transitions of the c-ZnS later. The longer range order of the as-grown c-ZnS crystal can also be probed by HRTEM. Fig. 1d displays the HRTEM image, the selective-area electron diffraction (SAED) pattern and the fast-Fourier transform (FFT) results of the as-grown ZnS crystal. The zone axis of the electron beam is along the [100] direction. Clear and obvious atomic sites arranged in the face-centre-cubic (FCC) style can be clearly seen in the HRTEM image of the ZnS in Fig. 1d. The lattice spacings of the (020) and (002) are similar and were determined to be ∼0.27 nm from the HRTEM image. This value matches well with the measured cubic lattice constant of a ≈ 5.4 Å from the XRD pattern of Fig. 1b. Both the SAED and FFT patterns in Fig. 1d are also similar and consist of the clear dotted features. This result also indicates the high crystalline quality of the as-grown ZnS crystals obtained by CVT growth.The high-quality epitaxial growth of optoelectronics devices may succeed but must start from an excellent substrate crystal. In particular, a high-quality ZnS substrate may directly contribute to the heterojunction carriers and photon absorption in the substrate and epilayer interface. This contribution is different from that of the other wide-band-gap substrates, such as sapphire and quartz. In order to evaluate the optoelectronic performance of the CVT c-ZnS substrate crystal, an initially-formed and hand-made substrate via diamond-saw cutting and diamond-paste polishing was also fabricated using the bulk ZnS in Fig. 1a. Experiments with PL, transmittance and CER were respectively carried out to assess the quality of the c-ZnS substrate. In general, the appearance of strong band-edge excitonic emission is a stringent index for high-crystallinity ZnS.1 To the best of our knowledge, only the high-quality ZnS thin films grown by the epitaxial methods of MOCVD and MBE, etc.15,16 can emit band-edge excitonic emission, although in the literature there is reported a very rare case of bulk ZnS with a longer range order that could emit band-edge excitonic luminescence at room temperature. Shown in Fig. 2a are the PL, transmittance and CER spectra of the c-ZnS substrate at 300 K. The morphology, size and transparency of the c-ZnS substrate are also displayed in Fig. 2b for contrast. The substrate plane is largely {100}, while the thickness of the substrate is about 600 μm and the transparency is ∼81% at 400 nm and ∼92% at 600 nm for visible light. The PL spectrum of Fig. 2a simultaneously shows a band-edge free-exciton (FX) emission at ∼3.68 eV as well as a lowered and broadened PL feature (ED) at ∼2.82 eV for the c-ZnS substrate. The strong band-edge emission indicates the high-crystalline quality of the ZnS substrate. We evaluate and discuss the low-temperature excitonic series emissions of the substrate later. The weakened and broadened PL feature at ∼2.82 eV may come from the contribution of a defect donor band formed by sulfur vacancies (VS) as indicated in the band-edge diagram in Fig. 2c. The occurrence of VS may be related to sulfur deficiency in the growth of the chalcogenide crystal. The PL intensity of ED also shows an enhancement at low temperature. For the CER measurement, the experimental data in Fig. 2a were analyzed by a first derivative Lorentzian line-shape function appropriate for the band-edge excitonic feature expressed as:21
 | (1) |
To further qualify the crystallinity of the initially-formed c-ZnS substrate, low-temperature and temperature-dependent PL measurements were performed near the band edge. Fig. 3a shows the low-temperature PL spectrum of the c-ZnS substrate at 10 K. The power density of the incident laser (λ = 266 nm) was reduced to ∼100 mW cm−2 via a neutral density filter (ND2.0). Four exciton-related peaks were detected and their energies were determined as FX = 3.799 eV, XX = 3.789 eV, DX0 = 3.782 eV and AX0 = 3.771 eV by fitting the PL spectrum to a Lorentzian line-shape function, with the results depicted in the lower part of Fig. 3a. The XX feature was assigned as a bi-exciton,23 while DX0 is a neutral-donor bound exciton and AX0 correlates with a neutral-acceptor-bound exciton in the c-ZnS.15–17 To further identify the origin of the series excitons FX, XX, DX0 and AX0, power-dependent PL measurements with changing the laser power from 10 W cm−2 (ND0, full power) to 100 mW cm−2 (ND2.0) were, respectively, carried out. Fig. 3b shows the variation of peak intensities of the series exciton emissions by the c-ZnS substrate at 10 K. Each PL curve of different powers (with fitting) was normalized to its maximum intensity to see the relative peak-value change. It could be noticed that the intensity of the XX emission was higher than that of DX0 at a low-power density (ND2.0), whereas it is weaker than that of the DX0 feature under the full-power condition (ND0) in Fig. 3b. The bi-exciton XX emission is more sensitive to the incident power change. It can be assigned as a surface-related exciton (SX) caused by the surface states in ZnS, as has also been found in previous PL band-edge emissions of ZnO.24
The temperature-dependent PL spectra of the c-ZnS substrate crystal using a laser power density of ND1.2 from 10 to 300 K are demonstrated in Fig. 4a. In addition to the series of the FX, XX(SX), DX0 and AX0 excitonic emissions, there is one extra emission of ESL ≈ 3.63 eV, and its phonon replicas 1LO ≈ 3.587 eV and 2LO ≈ 3.544 eV can be simultaneously detected in the PL spectrum at 10 K. The longitudinal optical phonon (LO-phonon) energy was determined as ∼43 meV, which is in good agreement with the most-prominent vibration peak of LO ∼ 350 cm−1 (ref. 25) detected in the Raman spectrum of the {100} plane of c-ZnS, as displayed in Fig. S1 (in the ESI†). The ESL emission at ∼3.63 eV was inferred to come from the shallow impurities of the acceptors and donors existing in the ZnS crystal, like that of a shallow-level emission (SLE) feature detected in a high-quality ZnS epilayer grown by pulse laser vaporization (PLV).1 The ZnS was claimed to have an unintentional shallow Na acceptor,1,15,16 and that possibly a little I2 transport agent (in CVT) could incorporate inside our ZnS crystal to form the iodine donor (i.e. replacing the S site). However, the power-dependent PL spectra of the ESL peak showed no energy shift with changing the laser power. This is maybe the origin of the occurrence of the shallow-level defect (impurity) emission of the ESL by a donor–acceptor recombination. Furthermore, as seen in Fig. 4a, the energy positions of the ESL and ESL-LO related emissions can be seen to be nearly invariant from 10 to 140 K. However, when T > 140 K, the ESL related emissions completely disappear due to thermal ionization. This behaviour agrees well with the SLE feature of a previous ZnS epilayer grown by PLV.1 The PL results of the c-ZnS in Fig. 2–4 verify that the bulk substrate of larger size (by CVT) still has a high crystallinity with respect to the other high-quality epitaxial thin films.
The temperature-dependent transition energies of the band-edge excitonic emissions FX, XX(SX), DX0 and AX0 analyzed from Fig. 4a for the c-ZnS substrate are, respectively, depicted as hollow squares, stars, hollow triangles and solid circles in Fig. 4b. As the temperature increases from 10 K, the intensity degradation of the bound-exciton complexes XX(SX), DX0 and AX0 in Fig. 4a becomes faster than that of the free-exciton emission FX. When T > 140 K, the bound-exciton complexes are ionized and the FX feature eventually dominates the band-edge PL emission in the c-ZnS (up to 300 K), such as is a general trend of semiconductors. The temperature dependence of the transition energy of FX can be analyzed using a Varshni-type equation:26
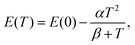 | (2) |
| ΘD ≈ 120Tm1/2A−5/6ρ1/3, | (3) |
 | (4) |
To further analyze the temperature dependence of the FX emission of the c-ZnS substrate, the PL temperature-intensity variation of Fig. 4a can be analyzed to obtain the activation energy Ea for the free exciton by using the Arrhenius formula as:30,31
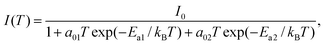 | (5) |
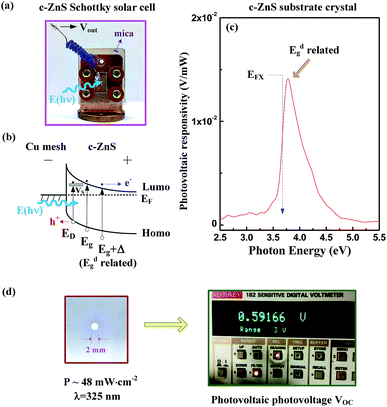
To reach the realization of the c-ZnS substrate, an initially-formed Schottky solar cell with a Cu mesh/c-ZnS substrate configuration was made and is shown in Fig. 5a. The size of the c-ZnS was 5 × 3 × 0.6 mm3. The substrate was attached onto a copper holder by sliver paste and then capped with a Cu mesh to form a Schottky junction on {100}. The voltage–current test revealed the Schottky diode behaviour with a forward cut-in voltage of ∼20 V and a reverse breakdown of ∼ −100 V. The representative band scheme of the Schottky-junction diode (with surface band bending) is depicted in Fig. 5b by taking into account the optical transitions detected in previous PL and CER measurements. On the c-ZnS may exist plenty of sulfur vacancies (VS) to form a probable n-type semiconductor. The contact can then form a metal-n Schottky diode for the Cu/ZnS junction. The direct gap between the lowest-unoccupied molecular orbital (Lumo, EC) and highest-occupied molecular orbital (Homo, EV) was ∼3.722 eV. When the photons E(hν) are incident into the junction, the defect and band-edge transitions ED, Eg and Eg + Δ may create electrons and holes, and then the carriers (e−, h+) are separated by the built-in electric field (Schottky band bending) to achieve a photovoltaic contribution. Fig. 5c shows the calibrated photovoltaic-responsivity spectrum of the Schottky solar cell (i.e. voltage/optical power) ranging from 2.5 to 5.5 eV. The spot size of the monochromatic light was about 100 × 100 μm2 and the optical power was measured by a thermal-type power meter. The spectrum in Fig. 5c shows a peak responsivity of ∼0.14 V mW−1 at the energy position near the direct band gap of ∼3.722 eV for the c-ZnS substrate. To test the electricity production of the initially-formed Schottky solar cell in the UV region, a He–Cd laser was utilized to act as the incident light source. As shown in the left part of Fig. 5d, the laser spot size was ∼2 mm and its power density was reduced to ∼48 mW cm−2. The right part in Fig. 5d clearly demonstrates a generated open-circuit voltage of VOC ∼ 0.6 V (i.e. with a short-circuit current, ISC ≈ 6 nA) will be created from the laser shinning in the initially-formed Schottky solar cell using the c-ZnS substrate. More electric power could be generated if we use multiple solar cell panels in connection to certain series-circuits designed for improving the output voltage and electricity production.
4. Conclusions
In conclusion, a high-quality ZnS substrate crystal with a longer range order and strong excitonic emission was grown by a CVT method using iodine as the transport agent. The as-grown crystals showed high transparency and high crystallinity with a crystal size of up to 1 cm. HRTEM, XRD, PL and CER measurements confirmed the cubic crystalline phase of the zinc sulfide. A complete series of band-edge excitonic emissions, including free exciton, bi-exciton (surface exciton), neutral-donor bound exciton and neutral-acceptor-bound exciton were detected in the low-temperature PL spectra, and their origins were respectively identified. The appearance of complete series excitonic emissions indicated the high crystal quality of the c-ZnS substrate. Analysis of the temperature-dependent PL spectra of the c-ZnS determined and identified the important physical and chemical parameters, including LO phonon energy, Debye temperature and the excitonic binding energy of the free excitons. All the successful analyses reflect the high crystalline quality of the zinc sulfide. The direct gap of c-ZnS was determined as 3.722 eV. An easily-formed Cu/c-ZnS Schottky solar cell was also attempted. A peak photovoltaic responsivity of 14 × 10−2 V mW−1 was detected near the spectral region of the direct band gap. The c-ZnS substrate solar cell is promising and more suitable for UV solar-energy conversion, especially in the ozone-free areas, such as outer-space (universe) electricity production.Acknowledgements
This work was sponsored by the financial support from the Ministry of Science and Technology of Taiwan under the grant no. MOST 104-2112-M-011-002-MY3.Notes and references
- R. Chen, D. Li, B. Liu and Z. Peng, Nano Lett., 2010, 10, 4956–4961 CrossRef CAS PubMed.
- K. B. Ozanyan, L. May, J. E. Nicholls, J. H. C. Hogg, W. E. Hagston, B. Lunn and D. E. Ashenford, J. Cryst. Growth, 1996, 159, 89–93 CrossRef CAS.
- A. Abounadi, M. D. Blasio, D. Bouchara, J. Calas, M. Averous, O. Briot, N. Briot, T. Cloitre, R. L. Aulombard and B. Gil, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 11677–11683 CrossRef CAS.
- S. Ves, U. Schwarz, N. E. Christensen, K. Syassen and M. Cardona, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 42, 9113–9118 CrossRef CAS.
- R. Pässler, E. Griebl, H. Riepl, G. Lautner, S. Bauer, H. Preis, W. Gebhardt, B. Buda, D. J. As, D. Schikora, K. Lischka, K. Papagelis and S. Ves, J. Appl. Phys., 1999, 86, 4403–4411 CrossRef.
- J. Nanda, S. Sapra, D. D. Sarma, N. Chandrasekharan and G. Hodes, Chem. Mater., 2000, 12, 1018–1024 CrossRef CAS.
- C. H. Ho, Y. J. Chen, H. W. Jhou and J. H. Du, Opt. Lett., 2007, 32, 2765–2767 CrossRef CAS PubMed.
- L. Guo, Y. L. Ji, H. Xu, P. Simon and Z. Wu, J. Am. Chem. Soc., 2002, 124, 14864–14865 CrossRef CAS PubMed.
- C. S. Tiwary, S. Saha, P. Kumbhakar and K. Chattopadhyay, Cryst. Growth Des., 2014, 14, 4240–4246 CAS.
- Y. Zhao, Y. Zhang, H. Zhu, G. C. Hadjipanayis and J. Q. Xiao, J. Am. Chem. Soc., 2004, 126, 6874–6875 CrossRef CAS PubMed.
- J. E. Northrup, J. Ihm and M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 1980, 22, 2060–2065 CrossRef CAS.
- X. Fang, Z. Wei, R. Chen, J. Tang, H. Zhao, L. Zhang, D. Zhao, D. Fang, J. Li, F. Fang, X. Chu and X. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 10331–10338 CAS.
- S. Kar and S. Chaudhurl, J. Phys. Chem. B, 2005, 109, 3298–3302 CrossRef CAS PubMed.
- B. Liu, Y. Bando, M. Liao, C. Tang, M. Mitome and D. Golberg, Cryst. Growth Des., 2009, 9, 2790–2793 CAS.
- Y. Kawakami, T. Taguchi and A. Hiraki, J. Cryst. Growth, 1988, 89, 331–338 CrossRef CAS.
- T. K. Tran, W. Park, W. Tong, M. M. Kyi and B. K. Wanger, J. Appl. Phys., 1997, 81, 2803–2809 CrossRef CAS.
- S. Nam, B. O and K. Lee, J. Appl. Phys., 1998, 84, 1047–1051 CrossRef CAS.
- C. Ma, D. Moore, J. Li and Z. L. Wang, Adv. Mater., 2003, 15, 228–231 CrossRef CAS.
- C. H. Ho, J. Mater. Chem., 2011, 21, 10518–10524 RSC.
- S. Biswas and S. Kar, Nanotechnology, 2008, 19, 045710 CrossRef PubMed.
- D. E. Aspnes, Modulation spectroscopy/electric field effects on the dielectric fuction of semiconductors, Handbook on Semiconductors 2, ed. M. Balkanski, North Holland, Amsterdam, 1980, ch. 4A, pp. 109–154 Search PubMed.
- A. I. Ryskin, L. G. Suslina, G. I. Khilko and E. B. Shadrin, Phys. Status Solidi B, 1972, 19, 875–884 CrossRef.
- N. Q. Leim, V. X. Quang, D. X. Thanh, J. I. Lee, A. K. Viswanath and D. Kim, Appl. Phys. Lett., 1999, 75, 3974–3976 CrossRef.
- J. Grabowska, A. Meaney, K. K. Nanda, J. P. Mosnier, M. O. Henry, J. R. Duclere and E. McGlynn, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 115439 CrossRef.
- O. Brafman and S. S. Mitra, Phys. Rev., 1968, 171, 931–934 CrossRef CAS.
- Y. P. Varshni, Physica, 1967, 34, 149–154 CrossRef CAS.
- J. M. Ziman, Electrons and Phonons: The Theory of Transport Phenomena in Solids, Clarendon Press, Oxford, 1960 Search PubMed.
- O. Madelung, Semiconductors-Basic Data, Springer Press, Berlin, 2nd rev edn, 1996 Search PubMed.
- C. H. Ho, Y. S. Huang, K. K. Tiong and P. C. Liao, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 16130–16135 CrossRef CAS.
- Y. Fang, L. Wang, Q. Sun, T. Lu, Z. Deng, Z. Ma, Y. Jiang, H. Jia, W. Wang, J. Zhou and H. Chen, Sci. Rep., 2015, 5, 12718 CrossRef CAS PubMed.
- K. A. Abel, H. Qiao, J. F. Young and C. M. J. van Veggel, J. Phys. Chem. Lett., 2010, 1, 2334–2338 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15150g |
| This journal is © The Royal Society of Chemistry 2016 |