The in vivo estrogenic modulatory effect of bisphenol A (BPA) on Oreochromis mossambicus and prevention of early maturation of ovary by conjugates of intracellular laccase and silica nanoparticles†
Abhijit Mannaa,
Shanmugam Geethaa,
Sembulingam Tamilzhalaganb and
Chinnaiah Amutha*a
aDepartment of Animal Behaviour and Physiology, School of Biological Sciences, Madurai Kamaraj University, Madurai – 625 021, India. E-mail: amuthaji@gmail.com; Fax: +91-452-2459139; Tel: +91-452-2458246
bDepartment of Genetics, School of Biological Sciences, Madurai Kamaraj University, Madurai – 625 021, India
First published on 10th October 2016
Abstract
Bisphenol-A (BPA) is a well-known endocrine disrupting chemical (EDC). Employing Oreochromis mossambicus (Tilapia) as a model organism, this study aimed to analyse the estrogenic modulatory effect of BPA and to prevent the early maturation of ovary by treating with conjugates of intracellular laccase and silica nanoparticles. Intracellular laccase was extracted from Trametes versicolor and purification of laccase was performed using fast protein liquid chromatography after running through ion exchange (DEAE-cellulose) followed by a gel filtration (P-6) column. Sol gel method was used to synthesize silica nanoparticles which were characterized by transmission electron microscopy, atomic force microscopy, dynamic light scattering, Fourier-transform infrared spectroscopy and X-ray diffraction. Silica nanoparticles were amino-functionalized and conjugated with intracellular laccase. Isothermal titration calorimetry showed that conjugation of intracellular laccase with silica nanoparticles was stable and the reaction was exothermic and spontaneous. Maximum effective concentration of BPA was found to be 100 ppm after short term exposure of Oreochromis mossambicus to BPA, at this concentration it leads to deleterious effects on liver (disruption of tissues), ovary (early maturation) and testis (antagonistic effect) which was more prominent in histopathological studies. Vitellogenin protein level and gene level expression analysis using FPLC and quantitative real time PCR showed that conjugates of laccase and silica nanoparticle were more effective in reducing the BPA concentration when compared to free laccase enzyme. Thus from the result it clear that conjugates of intracellular laccase and silica nanoparticle represents a promising tool to reduce the estrogenic effect of BPA by preventing the early maturation of ovary in O. mossambicus.
Introduction
Aquatic pollution leading to anthropogenic activities is a potential threat to aquatic ecosystems and their effects are largely uninvestigated. Transgenerational consequences or sex reversal is one of the greatest threats to aquatic organisms including fish on exposure to environmental estrogen-like endocrine disrupting chemicals (EDCs). EDCs are exogenous compounds which have the potential to disrupt the normal function of the endocrine system either by mimicking the body’s natural hormone level or by altering the natural production, metabolism or function of the hormone.1 BPA, a well known EDC, is ubiquitously present in aquatic ecosystems and a large number of studies have detected BPA contamination at μg L−1 to even low mg L−1 or mg kg−1 level.2 BPA is a commercially important chemical and predominantly used in the plastic industry, food and beverage containers. BPA has become one of the major toxic environmental pollutants due to its severe toxic effects on different living organisms within the range of 0.040–4 μM; at micromolar level (0.1–10 μM) it acts as an estrogenic and mutagenic agent.3 BPA, with its two benzene rings and two (4,4)-OH substituents, fits in the ER binding pocket and mimics as an estrogen. According to Gould et al., 1998,4 and Kuiper et al., 1998,5 BPA binds to both ERα as well as ERβ, with approximately 10-fold higher affinity to ERβ. BPA is also responsible for decline in sperm counts and abnormalities in the female reproductive system and is linked with the incidence of testicular, breast cancer and other medical disorders.6 BPA is moderately water soluble at ambient temperature (300 mg L−1) and the residue of BPA in water is due to incomplete polymerization. BPA has a relatively weak estrogenic potency compared to natural estradiol (E2) based on its binding affinity or activating capacity to estrogenic receptors (ERs) as found from in vitro assays.7,8 However, in vivo studies indicate that BPA can promote activities similarly to estrogen and can exhibit similar or stronger activity than E2.9 Due to the mimicking nature of BPA against natural estrogens as well as its competitive nature to bind with estrogen receptors, BPA can disrupt the normal function of the endocrine system and it is widely referred as a xenoestrogen and as an environmental estrogen. As with other chemicals, rivers and lakes have become major sources of BPA, as we can observe the impact of BPA on aquatic vertebrates, mainly fish, though also amphibians, reptiles and birds.10 In addition, it has been reported that BPA also has a potential antagonistic androgenic activity.11–13 Due to this deleterious effect on aquatic organisms there has been a great concern about their health and substantial development in the presence of BPA. Fish are often more sensitive to many toxins and are a convenient animal model and also a good representative in terms of aquatic ecosystem health. Oreochromis mossambicus (Tilapia) is an important common fish which is native to South Africa and commercialized in India. This species is available in fresh water and responds readily to alteration in its aquatic environment.14 Kang et al., 200715 reported that the main route of BPA exposure in fish is not through the diet but via inhalation through the gills. BPA in gills is not metabolized as efficiently as in the liver so that waterborne BPA will certainly lead to estrogenic modulation in fish, which includes the induction of vitellogenin (vtg), an egg yolk protein precursor in fish.15 Vitellogenin is a wide range molecular weight (250–600 kDa) calcium binding phospholipoglycoprotein, a precursor of egg yolk protein that is common to all oviparous vertebrates and essential for normal oocyte maturation. It is produced when there is estrogen stimulation by the liver and is released into the blood. Vtg production is normally restricted to mature females and is present at very low amount in males or sexually immature females. However, the exposure of fish to estrogenic compounds such as BPA can trigger vtg expression in males, since they have the vtg gene.16 Male fish also possess the hepatocyte estrogen receptor (ER) and results in vitellogenin gene expression when exposed to E2 or other estrogen mimetics. Its metabolism in males is slow, thus its presence in oviparous fish, particularly in males, makes it an ideal biomarker for studies on the effect of estrogenic EDCs on fish. BPA contamination in water leads to oxidative stress, destruction of macrophages (dysregulation of immune system) and early maturation of ovary in fish. So it is mandatory to remove or detoxify these hazardous EDCs from aquatic environments by means of bioremediation.Bioremediation is defined as a process which utilizes naturally occurring microbes to detoxify hazardous chemicals. One of the promising approaches to detoxify this EDC to a non-toxic compound is enzymatic bioremediation. Enzymes usually possess the characteristics of using these toxic chemicals as a metabolite in different organisms to fulfill their carbon, nitrogen and phosphorus requirements. BPA is biodegradable by several microbes and is metabolized by enzymes. Among BPA degrading enzymes, laccases are attractive candidates receiving increasing and significant attention because of their high stability, wide range of activity and the possibility to produce them in large-scale amounts.17–20 Laccases (p-diphenol–dioxygen oxidoreductases; EC1.10.3.2) are produced by the genus Trametes, a white-rot fungus assumed to be one of the main producers of laccases.21 T. versicolor produces laccase as a major ligninolytic enzyme and they are a group of blue multi-copper oxidases, catalyzing the oxidation reaction of a broad range of xenobiotics concomitantly with the reduction of molecular oxygen to water using electron shifting. This renders them very attractive compared to other enzymatic systems because no additional/expensive co-substrate or cofactor is required apart from atmospheric oxygen. Laccase enzymes usually contain four copper ions found in three active sites, participating in electron transfer from the substrate (T1 active site) towards oxygen (T2/T3 active sites). Crude and purified forms of laccases have been used to degrade BPA.22 The growing attention accorded to the removal of EDCs from environmental matrices makes oxidative enzymes attractive candidates and many of the undesirable limitations of free enzymes are overcome by the use of immobilized enzymes. In immobilized form, enzymes are more robust and more resistant to environmental changes, allowing easy recovery and multiple reuses.23 Nanocarriers are most often used as support materials for immobilization of the enzyme because of their unique properties, such as high surface area, shape retention, availability in different sizes, compositions, and characteristics after functional activation of the surface. Nanocarriers can maintain their stability even when very harsh chemical modifications are carried out.24–26 In our study, we used O. mossambicus as a model organism to study the estrogenic modulatory effect of BPA and preventing the early maturation of ovary by conjugates of intracellular laccase and silica nanoparticles.
Materials and methods
Purification and catalytic activity analysis of intracellular laccase enzyme
Biomass of seven days old Trametes versicolor culture was dissolved in 50 mL of sterile distilled water and sonicated twice at 80% power amplitude for 10 min with an interval of 2 min. During the sonication process samples were kept in ice in order to avoid overheating and to prevent denaturation. Whole sonicated samples were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C. Supernatant was taken and ammonium sulfate precipitation of intracellular laccase enzyme at 75% saturation level was performed. Precipitated sample was dialysed for 2 h, 4 h and overnight against sterile distilled water. After complete removal of salt using dialysis, the sample was allowed to run through a DEAE-anion exchange prepacked column using fast protein liquid chromatography.27 Tris–HCl (pH-8.0) buffer and a gradient of [0.1–1 M] NaCl was used during running the sample through fast protein liquid chromatography. Individual peaks appearing in the NaCl gradient line were collected using a fraction collector and concentrated using a vacuum evaporator. The fraction containing laccase was determined after incubating with laccase specific substrate ABTS; specific green colour formation confirmed the laccase–ABTS reaction. All the fractions containing laccase were collected and run through a P-6 gel filtration prepacked column using fast protein liquid chromatography with Tris–HCl (pH-8.0). Fractions containing intracellular laccase were run in SDS-PAGE for identifying specific molecular weight and zymogram analysis was performed in order to check the catalytic activity of the laccase enzyme. Intracellular laccase was allowed to run in 8% native PAGE and incubated in 10 mM ABTS solution for 15 min and waiting for specific colour formation.28
000 rpm for 15 min at 4 °C. Supernatant was taken and ammonium sulfate precipitation of intracellular laccase enzyme at 75% saturation level was performed. Precipitated sample was dialysed for 2 h, 4 h and overnight against sterile distilled water. After complete removal of salt using dialysis, the sample was allowed to run through a DEAE-anion exchange prepacked column using fast protein liquid chromatography.27 Tris–HCl (pH-8.0) buffer and a gradient of [0.1–1 M] NaCl was used during running the sample through fast protein liquid chromatography. Individual peaks appearing in the NaCl gradient line were collected using a fraction collector and concentrated using a vacuum evaporator. The fraction containing laccase was determined after incubating with laccase specific substrate ABTS; specific green colour formation confirmed the laccase–ABTS reaction. All the fractions containing laccase were collected and run through a P-6 gel filtration prepacked column using fast protein liquid chromatography with Tris–HCl (pH-8.0). Fractions containing intracellular laccase were run in SDS-PAGE for identifying specific molecular weight and zymogram analysis was performed in order to check the catalytic activity of the laccase enzyme. Intracellular laccase was allowed to run in 8% native PAGE and incubated in 10 mM ABTS solution for 15 min and waiting for specific colour formation.28
Cytotoxicity assay of intracellular laccase
To check the cytotoxic effect of intracellular laccase, AGS and MCF-12F cell lines were used and resazurin assay was performed in a 96-well plate (5000 cells per well) using DMEM (F12/HAM) culture media. After 24 h both cell lines were treated with various concentrations of laccase (50–2000 μg mL−1)29,30 and kept for 48 h incubation. 1 μM concentration of resazurin was added in all laccase treated AGS, MCF-12F cell lines and after 4 h of incubation the resorufin formation was measured fluorimetrically at 540 nm excitation and 590 nm emission.Synthesis, characterization and conjugation of silica nanoparticles with intracellular laccase
Determination of maximum effective concentration of BPA on Oreochromis mossambicus
Short term exposure of Oreochromis mossambicus to various concentrations of BPA ranging from 10 to 150 ppm was carried out to determine the maximum effective concentration of BPA (the concentration at which maximum effect was found). In this experiment 20 days of exposure was supplied to ten fish (equal male and female individuals) in different tanks in semi-natural conditions. After 20 days all treated fish were dissected and liver, ovary, testis were kept in PBS (pH-7.4) for 1 h, prior to transfer to 25% formalin solution and subsequent histopathological studies.Quantitative estimation of vitellogenin in individual test fish liver samples was also performed using a CUSABIO Grouper vitellogenin ELISA kit in order to evaluate the effective function of the laccase-nanoparticle conjugate in the reduction of endocrine disrupting effect of BPA in fish. Laccase-nanoparticle conjugate efficiency in the removal of BPA risk was statistically explained using one-way ANOVA.
In vitellogenin differential gene expression study, liver samples from control and treated fish were stored in RNAlater™ solution. 100 mg of liver sample was homogenized using a sterile micro-pestle, then 500 μL of TriZol was added and incubated at room temperature for 5 min. After incubation, 200 μL of chloroform was added and mixed vigorously. Then it was incubated at room temperature for 10 min. After incubation the sample was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min at 4 °C. Supernatant was transferred to a fresh microfuge tube and 500 μL of isopropanol was added. Then sample was incubated at room temperature for 10 min for precipitation. After incubation it was then centrifuged at 13
000 rpm for 15 min at 4 °C. Supernatant was transferred to a fresh microfuge tube and 500 μL of isopropanol was added. Then sample was incubated at room temperature for 10 min for precipitation. After incubation it was then centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C and the pellet was washed with 150 μL of 75% ethanol. The pellet was air-dried for 10 min and resuspended in 50 μL of DEPC treated water and stored at −80 °C. DNase treatment was given to the RNA and it was used as template for synthesis of first-strand cDNA. First-strand cDNA was constructed using revert aid H minus first-strand cDNA synthesis kit for all treated fish and the differential vitellogenin gene expression was quantified using ABI Prism 7000 q-PCR. Each 10 μL q-PCR reaction contained 5 μL of 2 × SYBr green mixes, 1 μL of cDNA, 2 μL of MilliQ, 1 μL of (10 pmol μL−1) forward and reverse vitellogenin gene specific primers. The thermocycle program included 94 °C (3 min), followed by 35 cycles of 94 °C (30 s), 55 °C for (30 s) and 72 °C (45 s).41 Each sample was evaluated in at least triplicate amplification reactions and each q-PCR run included control reactions containing no cDNA template. β-Actin was used as an internal control and the data was analyzed using the ΔΔCT method.42 The vitellogenin gene expression fold change was statistically analysed using one-way ANOVA.
000 rpm for 10 min at 4 °C and the pellet was washed with 150 μL of 75% ethanol. The pellet was air-dried for 10 min and resuspended in 50 μL of DEPC treated water and stored at −80 °C. DNase treatment was given to the RNA and it was used as template for synthesis of first-strand cDNA. First-strand cDNA was constructed using revert aid H minus first-strand cDNA synthesis kit for all treated fish and the differential vitellogenin gene expression was quantified using ABI Prism 7000 q-PCR. Each 10 μL q-PCR reaction contained 5 μL of 2 × SYBr green mixes, 1 μL of cDNA, 2 μL of MilliQ, 1 μL of (10 pmol μL−1) forward and reverse vitellogenin gene specific primers. The thermocycle program included 94 °C (3 min), followed by 35 cycles of 94 °C (30 s), 55 °C for (30 s) and 72 °C (45 s).41 Each sample was evaluated in at least triplicate amplification reactions and each q-PCR run included control reactions containing no cDNA template. β-Actin was used as an internal control and the data was analyzed using the ΔΔCT method.42 The vitellogenin gene expression fold change was statistically analysed using one-way ANOVA.
Results
Purification, analysis of catalytic activity and cytotoxicity of intracellular laccase enzyme
Purification of intracellular laccase was performed using DEAE & P-6 column. Single peaks of protein fraction from DEAE and P-6 columns (Fig. 1a and b) were concentrated and confirmed with substrate specificity reaction using ABTS as a substrate. Collected protein fraction gave significant green colour formation once incubated with ABTS and provided confirmation for the presence of laccase. Molecular weight determination for laccase also done by SDS-PAGE and it was obtained at 68 kDa range (Fig. 1c). The catalytic activity was again checked with zymogram (figure: Supplementary data-Sp-1a†). Distinct green colour bands appeared once incubated with ABTS which clearly showed that the purified laccase enzyme is catalytically active. In addition, cytotoxicity assay was performed with intracellular laccase enzyme to study if the enzyme possesses any cytotoxic effect in the environment. Resazurin assay with laccase enzyme in the presence of AGS & MCF-12F cell line clearly demonstrated that the enzyme did not posses any cytotoxic effect even at highest concentration (2000 μg mL−1) (figure: Supplementary data-Sp-1b, Sp-1c†). | ||
| Fig. 1 Enzyme purification: (a) laccase peak appearing in the DEAE column. (b) Laccase peak appearing in the P-6 column. (c) SDS-PAGE of intracellular laccase. | ||
Synthesis, characterization and conjugation of silica nano particles with intracellular laccase
The spherical nature of synthesized silica nanoparticles were clearly visible under TEM (Fig. 2a), apart from the TEM image, surface view and three-dimensional distributions of nanoparticles which were obtained from AFM (figure: Supplementary data-Sp-2a, Sp-2b†). X-Ray diffraction of silica nanoparticles confirmed their purity after comparisons with JCPDS file no: 76-0939 (figure: Supplementary data-Sp-2c†). The DLS data showed sizes around 500 nm with zeta potential of −20.73 (Fig. 2b and c). In FT-IR image, the Si–O–Si stretch was clearly seen at 1100–1090 cm−1 along with the hydroxyl stretch at 3429 cm−1 (figure: Supplementary data-Sp-2d†). Resazurin assay clearly indicated that even at highest concentration (2000 μg mL−1) silica nanoparticles did not show any cytotoxicity towards AGS and MCF-12F cell lines (figure: Supplementary data-Sp-2e, Sp-2f†).During the conjugation between silica nanoparticles with intracellular laccase, 200 μg of laccase was bonded with amino-functionalized nanoparticles, which was calculated after indirect measurement of unbounded protein from each wash solution using the Lowry method.22 FT-IR analysis was performed to check the occurrence of conjugation. The images clearly indicate that only in silica nanoparticles was there a peak at 3431 cm−1 due to the hydroxyl group but when it was amino-functionalised due to N–H stretching there was a shift from 3431 to 3444.98 cm−1, while for the modified nanoparticles conjugated with laccase the hydroxyl region was shifted more due to the hydroxyl group of enzyme. Clearly, from the FT-IR results conjugation occurred between the enzyme and modified nanoparticles (figure: Supplementary data-Sp-2g†). The conjugation and the thermodynamic parameters between modified nanoparticles and laccase were analyzed using isothermal titration calorimetry (ITC). From ITC, it was clear that the conjugation was exothermic in nature, and high positive value of entropy and negative value of enthalpy supported conjugation between enzyme and nanoparticles (Fig. 2d). After comparing with free laccase it was clear that after conjugation there was no negative effect observed in the activity of conjugates over a wide range of pH and temperature, but interestingly, at higher and lower temperature and pH, the activity of conjugates were higher than that of free laccase (figure: Supplementary data-Sp-2h†).
Comparative study of BPA degradation using free and nano-conjugated laccase
ESI-MS data (Fig. 3a–c) clearly indicated that the level of BPA degradation in the presence of laccase–nanoconjugates was significantly higher than that of free enzyme. The BPA peak was obtained at m/z 227 in the control, but in both treated samples peaks at m/z 453 and m/z 679 also appeared; from the MS database m/z at 453 was identified as 5,5-bis(4-hydroxyphenyl-1-methylethyl)biphenyl 2-2′-diol and m/z at 679 was 4-(2-(4-hydroxyphenyl)propan-2-yl)-2-(4-(2-(hydroxyphenyl)propan-2-yl)phenoxy)phenol, the modified dimer and trimer of BPA, respectively. The experiment was performed in a triplicate manner for calculating the reduction rates of BPA using free laccase and silica nanoconjugates. In case of all experimental replicates very similar percentages of degradation were observed; 50 and 35% reduction of BPA concentration was observed after the treatment with enzyme–nanoparticle conjugates and free laccase, respectively. Thus this shows that the conjugates of silica nanoparticles and laccase was more efficient in the reduction of BPA in comparison to free laccase enzyme. The modified dimer and trimer from the monomer of BPA are less toxic than BPA, having very little estrogenic activity, as the bigger structures and large steric hindrance can destabilize the binding of dimer or trimer with estrogen specific receptors.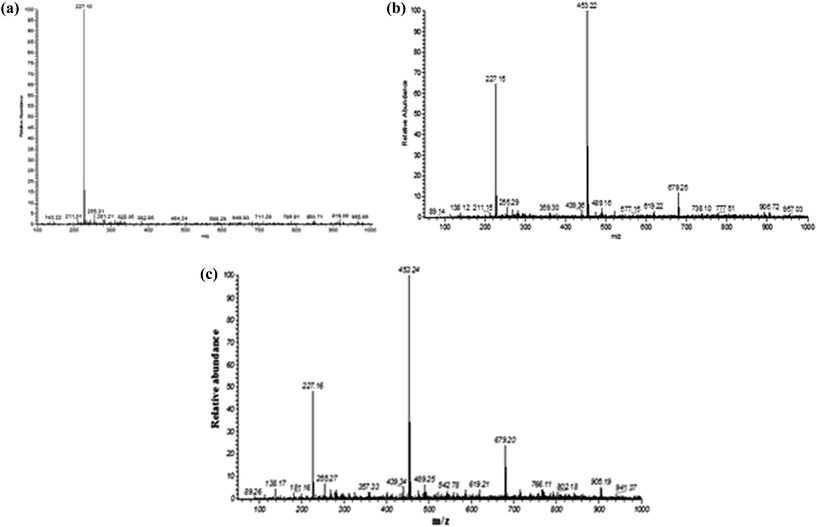 | ||
| Fig. 3 Comparative study of BPA degradation using free and nano-conjugated laccase: (a) control, (b) intracellular laccase treated, (c) conjugate treated. | ||
Determination of BPA maximum effective concentration on fish and comparative role of laccase and its conjugates
Histopathology images (figure: Supplementary data-Sp-3a, Sp-3b†) clearly revealed that 100 ppm concentration of BPA was the maximum effective concentration to exhibit maximum negative effect on liver (tissue rupture) and ovary (early maturation). Histopathology images showed (Fig. 4a–c) that BPA treated fingerling's ovary was almost as mature as sexually mature fish, whereas laccase treated individuals showed less maturation than solely BPA treated individuals, while significantly less maturation, almost similar to the control, was observed in the case of conjugate treated fish. BPA effect on liver was different relative to the ovary, because due to mimicking nature of BPA respect to estrogen it showed early maturation in ovary, but due to the acidic nature of phenolic BPA, it can rupture liver tissues. Histopathology images showed that in presence of only BPA, liver tissue rupture was devastating in comparison to all other treated samples and conjugate treated liver showed much less tissue rupture than free laccase treated fish. No change was observed in testis after treating with different combinations. | ||
| Fig. 4 Histopathology of liver, ovary and testis: (a) liver, (b) ovary, (c) testis (d) ESI-MS of liver sample. | ||
Due to the estrogen mimicking role of BPA, during fish dissection studies most prominent changes were observed. In only BPA treated fish, the ovary was full with complete mature eggs, in only laccase treated fish ovary contained eggs but these were not mature like solely BPA treated fish, but most interestingly, ovary of conjugate treated fish showed closest similarity with control fish (without BPA and treated with enzyme-nano only) (figure: Supplementary data-Sp-3c†). Both histopathology and dissection study confirmed that there was no early maturation effect in testis after treatment with different concentrations of BPA. Note: water used for exposure experiments showed no peak at m/z 227 corresponding to BPA (figure: Supplementary data-Sp-3d†). In this experiment also we have checked tissue samples of 10 fish (5 male and 5 female) in triplicate manner after sacrificing them. As supporting evidence different female fish liver were prepared for ESI-MS analysis and there was no peak at m/z 227 for non-BPA treated fish and or fish treated with enzyme-nanoparticles alone. Only BPA treated liver showed a prominent peak at m/z 227 indicating BPA deposition while conjugate treated liver samples showed very less amount of peak at m/z 227 than free laccase treated liver. Maximum reduction of m/z 227 was observed in conjugates which triggered enhancement of palmitic acid secretion (m/z 255) in both conjugates and in free laccase treated fish (Fig. 4d). The same effect was also observed in male fish liver (image not shown). Reduction rates of BPA in fish liver treated by free laccase or silica nanoconjugates was performed three times to accurately determine the degradation.
FPLC and q-PCR based detection of vitellogenin expression
FPLC results clearly depicted that only BPA treated female individual samples exhibited two prominent peaks (P-1, P-2), but in the presence of only laccase and conjugates treated samples, both P-1 and P-2 peak was reduced. It was observed that in conjugate treated samples reduction in both P-1 and P-2 peaks were more than free laccase treated samples. P-1 and P-2 was collected separately and ELISA was performed using vitellogenin specific kit. It was determined that the P-1 peak was due to vitellogenin (Fig. 5a–e). In male fish, in the case of BPA treated individuals a small peak (figure: Supplementary data-Sp-4a, Sp-4b, Sp-4c, Sp-4d, Sp-4e†) was observed but other treated samples did not show any peak for vitellogenin. After quantitative estimation of treated samples, it was clear that vitellogenin protein expression was highest in both BPA treated female and male samples. Vitellogenin protein from FPLC was concentrated using a vacuum evaporator and in SDS-PAGE (figure: Supplementary data-Sp-4f†) the band intensity of BPA treated female liver sample showed the most prominent band followed by only enzyme treated individuals, conjugate treated individuals and negative controls, though in female fingerlings as a whole the level of vitellogenin synthesis is usually low and the same effect was observed in controls (without BPA treated) along with conjugate treated individuals, a consequence of which was that the band in SDS-PAGE was very faint. In case of males, vitellogenin secretion usually very much lower, and all treated combinations did not show any band in SDS-PAGE, though quantitative estimation by ELISA showed that BPA treated individuals showed maximum vitellogenin secretion followed by laccase, conjugate treated individuals and other two negative controls. In both male and female samples after performing ELISA (Fig. 5f), by analyzing with one way ANOVA (P < 0.001) a statistically significant difference was observed between conjugates and free laccase as well as with solely BPA treated individuals and this indicates that conjugates can effectively reduce the estrogenic effect of BPA more than free laccase.Differential expressions of vitellogenin gene on exposure of BPA and also after treatment with conjugates, free laccase, along with negative controls were analyzed using quantitative real time PCR. The vitellogenin gene expression fold change was observed by reference with internal control β-actin and the data were analyzed using ΔΔCT method. In the case of male fish, results showed that there was a maximum expression of vitellogenin when exposed to BPA but fish which were treated with conjugates showed decreased vitellogenin gene expression similarly to control male fish, because usually there is a minimal level of vitellogenin gene expression at the male fingerling stage. In the case of female fish, vitellogenin gene expression plays a very significant role in vitellogenisis by producing vitellogenin protein. BPA exposed female fish showed maximum level of vitellogenin gene expression but in conjugate treated fish there was a considerable reduction in gene expression compared to free laccase enzyme treated fish. Though the vitellogenin gene expression was not reduced like that of negative control in female fish, it was almost the same in the case of male fish, because being an egg precursor protein, expression of vitellogenin is usually higher in female than male fish, and analysis by one-way ANOVA (P < 0.001) showed prominent statistical significant difference between the function of laccase conjugates with respect to solely BPA treated individuals in both male and female fish. Statistical analysis clearly indicated that conjugates can significantly reduce the toxicity of BPA relative to free laccase (Fig. 5g).
Discussion
Laccase is a widely used oxido reductase enzyme which has a significant role in many industrial and environmental aspects but the free laccase enzyme readily loses its activity under some conditions and also in the presence of inhibitors. To overcome this limitation enzyme immobilization is widely preferred. In some cases, larger size immobilizing substratum can alter enzyme conformation and leads to loss of its activity. Immobilization of enzyme using nanoparticles as a substratum is a promising route to retain the enzyme activity. Nanoparticles have a large surface area and a large number of enzyme molecules can attach on the surface. Due to the higher exposure of enzyme active sites it can react with a large number of substrates in a stable manner. In this study we have tried to enhance the activity of laccase in order to remove or detoxify the harmful environmental toxicant BPA. Intracellular laccase obtained from Trametes versicolor will be in higher concentration due to less protease activity. Before employing laccase enzyme and silica nanoparticles in bioremediation it is mandatory to check the cytotoxicity effect of enzyme and nanoparticles, though from resazurin assay it is clear that neither possesses any cytotoxicity, even at highest concentration (2000 μg mL−1). When we treated fish with only enzyme and nanoparticles to investigate any negative role of silica nanoparticles in living systems, histopathology along with fish dissection clarifies that laccase as well as silica nanoparticles do not have any negative role on fish [Fig. 4a–c and Supplementary data-Fig. 3c†]. A successful bioremediation is one which yields end products which are completely safe or of very low toxicity and are eco-friendly, but in most cases bioremediation of toxic pollutants such as BPA failed to give the expected result. Bioremediation of BPA with laccase and silica nanoparticles, however, modified this toxic substance into the dimer and trimer which are much less toxic and do not act as potent environmental estrogens like BPA, hence the estrogenic modulatory effect of BPA has been greatly reduced by laccase and silica nanoparticle conjugates. Histopathology study clearly indicated that conjugates can reduce the maximum effective concentration of BPA. Presence of conjugates can reduce BPA to a safe level that does not affect the liver or ovary. Dissection of conjugate treated fish reveal ovary almost the same as control fish ovary. Histopathology and dissection results were then correlated from ESI-MS data; in conjugate treated fish liver the BPA peak was very low because it was degraded below its toxic level by conjugates. In solely BPA treated fish BPA concentration was above the liver detoxification level, and as a result liver damage was maximum and early maturation also maximum in ovary. Enzyme treated individuals showed some reduced concentration of BPA but not as much as conjugate treated individuals – only conjugate treated fish showed maximum effects compared to free enzyme treated individuals. Another important result was increasing concentration of palmitic acid in conjugate treated and only laccase treated fish was observed which leads to protection of the liver because palmitic acid is a saturated fatty acid having antioxidant activity,43 but it was not clear why palmitic acid was not secreted in higher amount in presence of higher BPA concentration, or why it was secreted in higher concentration after reduction of BPA concentration. A possibility might be that a higher concentration of BPA can down-regulate the expression of the palmitic acid gene and after reduction of BPA stress the palmitic acid gene can be expressed more. Vitellogenin peak was observed in FPLC and the band intensity of vitellogenin monitored using SDS-PAGE indicated that the concentration of vitellogenin in solely BPA treated fish was high but significantly for conjugate treated individuals the vitellogenin peak and band intensity were similar to control individuals. Using quantitative RT-PCR it was also determined that in solely BPA treated individuals, vitellogenin gene expression was very high but in conjugate treated individuals much lower expression of vitellogenin level was observed than in laccase treated individuals. From the above study one important outcome is that due to the estrogenic activity of BPA, female fish mature earlier but being an antagonist of androgen, male fish do not become mature, and as a result due to continuous exposure of BPA, female fish will not fertilized by the immature male fish.BPA is a kind of silent killer for our environment. Laccase immobilization techniques, however, may give a suitable and proper remediation against BPA toxicity. The raw materials required for protein purification are very cheap as is laccase enzyme (mainly from widely available white rot fungus) and large-scale production using a fermenter is also very easy. Additionally, the raw materials needed for silica nanoparticle synthesis are also very cheap, non-toxic and stable. Laccase and nano particle conjugation require APTS and glutaraldehyde which are also very common and cheap. Accordingly, our approach is cost efficient for bioremediation purposes.
Conclusion
Owing to continuous pollution of BPA in the aquatic environment, there is a danger of extinction of not only fish but most organisms from BPA contaminated sites. After intense research and understanding we propose the use of laccase-nanoconjugates against the toxic effect of BPA, for instance in small scale bioremediation at discharge sites of industries which release BPA and in application in stagnant regions to reduce the concentration of BPA below the toxic level using immobilized laccase.Acknowledgements
This research was supported by Department of Biotechnology-Interdisciplinary programme in life science (DBT-IPLS PhD programme, India). We are very much thankful to Dr G. Kumaresan for helping in cytotoxicity analysis.References
- National Institute of Environmental Health Science, Endocrine Disruptors, 2010 Search PubMed.
- M. Yang, W. Qiu, B. Chen, J. Chen, S. Liu, M. Wu and K. J. Wang, Environ. Sci. Technol., 2015, 49, 1888 CrossRef CAS PubMed.
- S. Saiyood, S. Vangnaia, P. Thiravetyan and D. Inthorn, J. Hazard. Mater., 2010, 178, 777 CrossRef CAS PubMed.
- J. C. Gould, L. S. Leonard, S. C. Maness, B. L. Wagner, K. Conner, T. Zacharewski, S. Safe, D. P. McDonnell and K. W. Gaido, Mol. Cell. Endocrinol., 1998, 142, 203 CrossRef CAS PubMed.
- G. G. Kuiper, J. G. Lemmen, B. Carlsson, J. C. Corton, S. H. Safe, P. T. Van Der Saag, B. van der Burg and J. A. Gustafsson, Endocrinology, 1998, 139, 4252 CAS.
- C. Arboleda, H. Cabana, E. De Pril, J. Peter Jones, G. A. Jiménez, A. I. Mejía, S. N. Agathos and M. J. Penninckx, ISRN Biotechnol., 2013, 2013, 1 CrossRef PubMed.
- K. W. Gaido, L. S. Leonard, S. Lovell, J. C. Gould, D. Babaï, C. J. Portier and D. P. McDonnell, Toxicol. Appl. Pharmacol., 1997, 143, 205 CrossRef CAS PubMed.
- M. Marques, L. Laflamme, I. Benassou, C. Cissokho, B. Guillemette and L. Gaudreau, BMC Cancer, 2014, 14, 524 CrossRef PubMed.
- M. P. Alonso, A. B. Ropero, S. Soriano, A. M. García, C. Ripoll, E. Fuentes, I. Quesada and A. Nadal, Mol. Cell. Endocrinol., 2012, 355, 201 CrossRef PubMed.
- C. Laura and F. Elena, Dose-Response, 2015, 13, 1 Search PubMed.
- P. Sohoni and J. P. Sumpter, J. Endocrinol., 1998, 158, 327 CrossRef CAS PubMed.
- H. J. Lee, S. Chattopadhyay, E. Y. Gong, R. S. Ahn and K. Lee, Toxicol. Sci., 2003, 75, 40 CrossRef CAS PubMed.
- H. Sun, L. C. Xu, J. F. Chen, L. Song and X. R. Wang, Food Chem. Toxicol., 2006, 44, 1916 CrossRef CAS PubMed.
- K. C. Chitra, Indian J. Sci. Res., 2014, 3, 221 Search PubMed.
- J. H. Kang, D. Aasi and Y. Katayama, Crit. Rev. Toxicol., 2007, 37, 607 CrossRef CAS PubMed.
- J. P. Sumpter and S. Jobling, Environ. Health Perspect., 1995, 103, 173 CrossRef CAS PubMed.
- J. P. J. Cabana and S. N. Agathos, Eng. Life Sci., 2007, 7, 429 CrossRef.
- Laccase in Handbook of Metalloprotein, ed. G. J. Davies, V. Ducros, R. Messerschmidt, K. Huber and T. P. Wieghardt, Wiley-Interscience, Weinheim, 2001 Search PubMed.
- L. C. Octavio, P. P. M. C. Irma, B. R. J. Ricardo and V. O. Francisco, Adv. Agric. Food Biotechnol., 2006, 323 CAS.
- L. Gianfreda, F. Xu and J. M. Bollag, Biorem. J., 1999, 3, 1 CrossRef CAS.
- I. Stoilova, A. Krastanov and V. Stanchev, Adv. Biosci. Biotechnol., 2010, 1, 208 CrossRef CAS.
- P. Giardina, V. Faraco, C. Pezzella, A. Piscitelli, S. Vanhulle and G. Sannia, Cell. Mol. Life Sci., 2010, 67, 369 CrossRef CAS PubMed.
- C. F. Thurston, Microbiology, 1994, 140, 19 CrossRef CAS.
- E. M. Amman, C. A. Gasser, G. Hommes and P. F. X. Corvini, Appl. Microbiol. Biotechnol., 2014, 98, 1397 CrossRef PubMed.
- Y. Liu, Z. Zeng, G. Zeng, L. Tang, Y. Pang and Z. Li, Bioresour. Technol., 2012, 115, 21 CrossRef CAS PubMed.
- A. P. M. Tavares, O. Rodríguez, M. Fernández-Fernández, A. Domínguez, D. Moldes, M. A. Sanromán and E. A. Macedo, Bioresour. Technol., 2013, 131, 405 CrossRef CAS PubMed.
- B. Chefetz, Y. Chen and Y. Hadar, Appl. Environ. Microbiol., 1998, 64, 3275 Search PubMed.
- M. E. Arias, M. Arenas, T. Rodriguez, J. Solveri, A. S. Ball and M. Hernandez, Appl. Environ. Microbiol., 2003, 69, 1953 CrossRef CAS PubMed.
- A. D. S. Christie and S. Shanmugam, Res. J. Pharm., Biol. Chem. Sci., 2012, 3, 912 Search PubMed.
- G. Ravikumar, D. Gomathi, M. Kalaiselvi, K. Devaki and C. Uma, Int. J. Res. Pharm. Biomed. Sci., 2013, 4, 1148 Search PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- I. A. B. Rahman and V. Padavettan, J. Nanomater., 2012, 2012, 1 CrossRef.
- M. Arturo Lopez-Quintela, Curr. Opin. Colloid Interface Sci., 2003, 8, 137 CrossRef.
- I. A. M. Ibrahim, A. A. F. Zikry and A. M. Sharaf, J. Am. Sci., 2010, 6, 985 Search PubMed.
- W. C. Hinds, Aerosol Technology: Properties, Behaviour and Measurement of Airborne Particles, Wiley-Blackwell, 1999, ISBN: 978-0-471-19410-1 Search PubMed.
- P. Galliker, G. Hommes, D. Schlosser, P. F. Corvini and P. Shahgaldian, J. Colloid Interface Sci., 2010, 349, 98 CrossRef CAS PubMed.
- H. Lowry, N. J. Rosebrough, A. L. Farr and R. L. Randall, J. Biol. Chem., 1951, 193, 265 Search PubMed.
- S. K. S. Patel, J. Microbiol. Biotechnol., 2014, 24, 639 CrossRef CAS PubMed.
- C. A. Staples, P. B. Dorn, G. M. Klecka, S. T. O’Block, D. R. Branson and L. R. Harris, Chemosphere, 2000, 40, 521 CrossRef CAS PubMed.
- S. E. Bartell and H. L. Schoenfuss, ISRN Toxicology, 2012, 2012, 942804 CrossRef PubMed.
- M. M. Esterhuysea, M. Venterb, N. Veldhoenc, C. C. Helbingc and J. H. vanWyk, J. Steroid Biochem. Mol. Biol., 2009, 117, 42 CrossRef PubMed.
- K. J. Livak and T. D. Schmittgen, Methods, 2001, 25, 402 CrossRef CAS PubMed.
- K. H. Cho, J. K. Hong and K. T. Lee, J. Med. Food, 2010, 13, 99 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15098e |
| This journal is © The Royal Society of Chemistry 2016 |


