3D graphene–carbon nanotube–nickel ensembles as anodes in sodium-ion batteries†
Abstract
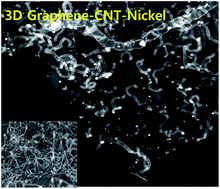
Sodium ion batteries (SIBs) are fast emerging as an attractive low-cost substitute in applications such as smart grids and large scale energy storage. But traditional carbon anodes used in SIBs face many practical difficulties due to the fact that the sodium ion is 55% larger than its lithium counterpart. Therefore, anodes in SIBs require special architectures to accommodate the large sodium ions without compromising other essential attributes like conductivity and surface area. In this manuscript, we report the synthesis of three dimensional, 3D mesoporous, N-doped graphene–carbon nanotube (G–Ni@NCNT) networks as anodes in SIBs. The graphene–carbon nanotube–nickel ensemble was synthesized by facile catalytic transformation of isocyanate treated graphene oxide under microwave irradiation. When applied as electrodes in sodium-ion batteries, our 3D graphene–carbon nanotube hybrids show a high specific capacitance of 447 mA h g−1, good rate capability and still retain 98.4% of the initial capacitance even after 150 cycles. The carbon nanotubes anchored on the graphene surface act as spacers and increase the electrolyte-accessible surface area whereas the defects generated by substitution doping of nitrogen on graphene and CNT provide good anchoring points for sodium ion retention.

 Please wait while we load your content...
Please wait while we load your content...