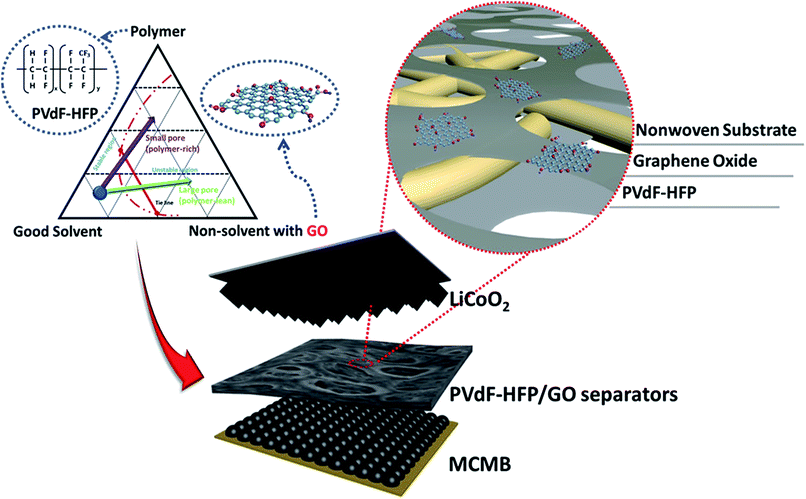
PVdF-HFP/exfoliated graphene oxide nanosheet hybrid separators for thermally stable Li-ion batteries†
Yunah Choia,
Kan Zhanga,
Kyung Yoon Chungb,
Dong Hwan Wang*c and
Jong Hyeok Park*a
aDepartment of Chemical and Biomolecular Engineering, Yonsei University, 50 Yonsei-ro, Seodaemun-gu, Seoul 120-749, Republic of Korea. E-mail: lutts@yonsei.ac.kr
bCenter for Energy Convergence Research, Korea Institute of Science and Technology, Hwarang-ro 14-gil 5, Seongbuk-gu, Seoul 136-791, Republic of Korea
cSchool of Integrative Engineering, Chung-Ang University, 84 Heukseok-Ro, Dongjak-gu, Seoul 156-756, Republic of Korea. E-mail: king0401@cau.ac.kr
First published on 15th August 2016
Abstract
We report novel poly(vinylidene fluoride-co-hexafluoropropylene) (PVdF-HFP)/graphene oxide (GO) nanocomposite separators for lithium secondary batteries. During phase inversion of PVdF-HFP which is the origin of pore generation, GO nanosheets can be incorporated into the PVdF-HFP skeleton. The battery performances of the cells with the PVdF-HFP/GO nanocomposite separators were greatly improved compared to the pure PVdF-HFP based one. In addition, the thermal stability of the nanocomposite separators is also greatly enhanced by the addition of GO, in which the battery cells with composite separators that are thermally annealed at 150 and 180 °C function well. The inclusion of 2-dimensional GO nanosheets could also enhance the tensile strength of neat PVdF-HFP separators, leading to mechanically stable polymer separators.
Introduction
Lithium ion batteries (LIBs) have been widely considered as the most powerful energy storage system for a number of portable electronic devices, electric vehicles and emerging smart grids because of their important advantages, including high energy density, long cycle life, and low self-discharging. LIBs generally consist of the cathode, anode, electrolytes, and separators, of which the separator plays the key role of maintaining electrical isolation between the anode and the cathode while acting as the electrolyte reservoir for the transport of ions.1–5 Most of the separators in commercialized LIBs are made of polyethylene (PE) owing to the uniform pore size, high mechanical strength, and electrochemical stability of PE.6 However, due to the high cost of PE separators and lack of thermal and dimensional stability, phase inversion-based poly(vinylidene fluoride-co-hexafluoropropylene) (PVdF-HFP) polymer separators have been noted as an effective way to prepare porous membranes.7 Even though tailored pore structure and enhanced thermal stability have been retained in PVdF-HFP separators using the phase inversion method8 and adding nanomaterials,9–11 LIB performance and stability exceeding conventional PE separators are still challenging issues.Meanwhile, graphene oxide (GO) is made of a monolayer of graphite oxide and almost gained by the oxidation of graphite.12 GO is hydrophilic and easy to disperse in DI water. Because GO has both sp2 and sp3 domains, it expands the more kinds of interactions that can react with the surface.13 Besides, due to these advantages in view of mechanical and thermal stability, large surface area and low interfacial resistance, GO is attractive material to be applied for preparing separators of LIB.14 Recently, GO has been used to membranes for various rechargeable batteries. Huang et al. manufactured the ultrathin GO membrane and achieved reduced the cyclic capacity decay as well as the diffusion of polysulfides through the membrane in lithium–sulfur batteries.15 Also, Chu et al. produced the GO/Nafion composite membrane to increase the energy efficiency of vanadium redox battery system.16
For the first time, we report PVdF-HFP/GO separators with enhanced thermal stability, in which 2D graphene oxide (GO) nanosheets were incorporated into PVdF-HFP skeletons (Scheme 1). In the PVdF-HFP separators, Li-ions in an electrolyte can move through the pores generated by interconnected PVdF-HFP skeletons. The covalent oxygen functional groups in GO make it remarkably compatibility with polar solvents. Then, the added GO nanosheets in the PVdF-HFP framework can improve the wettability with polar solvents for additional Li-ion transport pathways, thus help improving the separator performances. Furthermore, incorporating PVdF-HFP and GO nanosheets can maintain their original pore structures, even up to 170 °C, which means the nanocomposite separator has great thermal stability compared to pure PVdF-HFP separators.
Experimental
Synthesis of graphene oxide
Graphene oxide was obtained by Hummer's method.17 First, graphite powder (1 g, purity 99.9999%, Alpha Aesar) was dispersed in cold, strong sulfuric acid (25 mL, 98 wt%, ice bath) with 1 g of NaNO3, and potassium permanganate (KMnO4, 3 g) was slowly added with continuous cooling and stirring to keep the temperature below 20 °C. The ice bath was replaced by a water bath, and the mixture was heated to 35 °C for 1 h to release gas under continuous stirring. Then, deionized water (50 mL) was added slowly, which produced a rapid increase in the solution temperature to a maximum of 98 °C. The reaction was held for 12 h to increase the oxidation degree of the GO product. The resultant bright-yellow suspension was obtained by adding distilled water (140 mL), followed by hydrogen peroxide solution (H2O2, 30%, 3 mL). The resultant product was separated by centrifugation and was washed with 200 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 HCl solution and water until the pH reached 7. The powder was then dried in vacuum at room temperature. Chemical reagents other than graphite powder were purchased from Aldrich and were used without further purification.
10 HCl solution and water until the pH reached 7. The powder was then dried in vacuum at room temperature. Chemical reagents other than graphite powder were purchased from Aldrich and were used without further purification.
Preparation of the PVdF-HFP/graphene oxide nanocomposite membrane
The PVdF-HFP/GO nanocomposite membranes were obtained as follows. First, we used to PET nonwoven as substrate (thickness = 18 μm). And the coating solution was prepared by dissolving two types of PVdF-HFP with different HFP concentrations (12 wt% for “PVdF A” and 6 wt% for “PVdF B”) in an acetone solvent. The two amounts of PVdF-HFP were dissolved in the solvent with various mixing ratios (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (PVdF A
3 (PVdF A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVdF B by mass)). After the PVdF-HFP was fully dissolved in acetone solvent by stirring, we added GO-dispersed water in the coating solution. GO-dispersed water solution prepared to disperse GO into water, where the GO content was varied from 0 to 0.0132 wt% (GO/PVdF-HFP). To well disperse GO in water, the solution was ultrasonicated for 15 min by using a ULH 700S Sonosmasher (Ulsso Hightech, Korea). The resulting GO-dispersed water at fixed 4% (i.e., 4% GO-dispersed water/(GO-dispersed water + acetone) by mass) was dropped slowly into coating solution by stirring. To eliminate the impurities, the PET nonwoven substrate was cleansing by acetone. The PVdF-HFP/GO polymer solutions were coated onto PET nonwoven-based separators by dip-coating, followed by drying at 25 °C and 40% relative humidity. The PVdF-HFP/GO polymer solution-immersed PET nonwovens were completely dried at room temperature.
PVdF B by mass)). After the PVdF-HFP was fully dissolved in acetone solvent by stirring, we added GO-dispersed water in the coating solution. GO-dispersed water solution prepared to disperse GO into water, where the GO content was varied from 0 to 0.0132 wt% (GO/PVdF-HFP). To well disperse GO in water, the solution was ultrasonicated for 15 min by using a ULH 700S Sonosmasher (Ulsso Hightech, Korea). The resulting GO-dispersed water at fixed 4% (i.e., 4% GO-dispersed water/(GO-dispersed water + acetone) by mass) was dropped slowly into coating solution by stirring. To eliminate the impurities, the PET nonwoven substrate was cleansing by acetone. The PVdF-HFP/GO polymer solutions were coated onto PET nonwoven-based separators by dip-coating, followed by drying at 25 °C and 40% relative humidity. The PVdF-HFP/GO polymer solution-immersed PET nonwovens were completely dried at room temperature.
Physicochemical and electrochemical characterization of the PVdF-HFP separator
Scanning electron microscopy (SEM) images of the separators were taken with a field emission scanning electron microscope (FE-SEM, JSM-7600F, Japan). SAXS analysis was conducted as follows: SAXSess camera (Anton-Paar, Graz, Austria) was used and X-ray generator (Philips, PW 1730/10) operated at 40 kV and 50 mA with a sealed-tube Cu anode. Divergent polychromatic X-ray beam was aligned to collimated line-shaped beam of Cu Kα radiation (λ = 0.154 nm) by using a Göbel mirror. Imaging-plate detector (model Fuji BAS1800 from Raytest, Straubenhardt, Germany) reported the 2D scattering pattern. Also, it was incorporated a one-dimensional scattering function I(q) utilizing SAXS Quant software (Anton-Paar). The scattering vectors q obtained by this constitution ranged from 0.01 to 3 nm−1. The thickness of PVdF-HFP/exfoliated GO samples was 25 μm and were analyzed at 25 °C. Then, thermal properties of the samples were evaluate by using thermogravimetric analysis (TGA 6000, Seiko Instruments). The modified separators were heated from 25 to 600 °C at 10 °C min−1 with a nitrogen atmosphere. The ionic conductivity (σ) of the separator was conducted by AC impedance spectroscopy (IM6-6eX) ranged from 0.01 to 100 kHz.20 The porosity analysis of the separator was conducted mercury intrusion porosimetry (Autopore IV 9500, Micomeritics, Spanish). The mechanical properties of the membrane were determined using a universal tensile machine (Nexygen Plus model LR30K tester, Lloyd Instruments, England). The test specimens were 5 mm wide and approximately 25 μm thick, the grip distance was 50 mm and the cross-head speed was 10 mm per min.To test the cell performance, a unit cell was assembled. The electrolyte for the electrochemical performance was 1 M LiPF6 and a mixture of ethylene carbonate (EC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) (EC/DEC/EMC = 1/1/1 wt/wt/wt, PuriEL, Soulbrain Co., Ltd, Korea). The lithium coin cell (2032 battery type) was formed by sandwiching the composite separator between a 94 wt% mesocarbon microbead (MCMB) anode (6 wt% Kynar 741 polymeric binder) and a 90 wt% LiCoO2 cathode (4 wt% PVdF binder and 6 wt% Super-P) and then activated by filling the space with liquid electrolyte. For the charge–discharge test, cells were charged to 4.3 V at 0.2C and then were discharged to 3.0 V at various C-rates. The charge process was cut off at 20% of the initial constant current.
Results and discussion
To incorporate 2D GO nanosheets into PVdF-HFP polymers, two different solutions were prepared and mixed, as described in the Experimental section: PVdF-HFP in acetone and GO in water. Because of the small content of non-solvent (water) in good-solvent (acetone) the mixed PVdF-HFP polymer solution is homogeneous. When nonwoven (18 μm thick, wet-laid) (Fig. S1A, ESI†) was dip-coated with the mixed solution, acetone with a lower boiling point evaporated faster than water, which induced phase separation of the coated PVdF-HFP polymer (Fig. 1A and B). During pore generation, well-dispersed GO nanosheets were also incorporated into the PVdF-HFP polymer.The exfoliated GO suspension was deposited on pristine silicon wafer and characterized via scanning electron microscopy (SEM). As shown in Fig. S2A,† the formed crumpled structures had relatively low surface tension, which caused them to shrink inwardly. When the exfoliated GO was dropped on the top of the grid for transmission electron microscopy (TEM), it exhibited silk veil waves that were not entangled with each other (Fig. S2B†). The phenomenon could be ascribed by its amphipathicity with an edge-to-center distribution of hydrophilic and hydrophobic domains.18,19 The high-resolution TEM taken from the exfoliated GO suggests a few-layer structure accompanied by a tailored edge (Fig. S2C†). The layer number of the exfoliated GO was determined by atomic force microscopy (AFM) combined with the XRD pattern. The strong 002 peak at 10.5° indicates an interlayer spacing of 8.4 Å. The average thickness of ∼1.6 nm (Fig. S2D†) indicates average double-layer GO sheets.
The effectiveness of adding GO nanosheets on the surface morphologies of the PVdF-HFP separators was observed. The different HFP content in the PVdF-HFP separators resulted in a mixed porosity of large (∼several micrometers) and small pores (∼submicrometers) during the phase inversion. As observed in Fig. 1A and B, the addition of GO nanosheets to the PVdF-HFP film did not have a significant influence on the macroscopic pore generation. Moreover, the added GO nanosheets were not easily observed by scanning electron microscope (SEM). To verify the presence of GO nanosheets in the PVdF-HFP film, SAXS patterns of the PVdF-HFP separator with increasing contents of GO nanosheets were investigated (Fig. 1C).
Commonly, the crystallization of semicrystalline polymers can be disrupted by adding an increasing content of inorganic nanoparticles.20–36 Additionally, the SAXS data showed that this morphology transition of the film is due to the existence of GO nanosheets. The original SAXS peak of the PVdF-HFP film at q = 0.3 was gradually weakened with increasing contents of graphene oxide nanosheets, indicating that the domain structure of the blends was disordered. Moreover, with increasing GO nanosheets contents in the separators, the color of the separators changed gradually from white to grey, as shown in Fig. S3.† The thermogravimetric (TGA) analysis showed that the thermal stability of the polymer was enhanced when GO nanosheets were incorporated into the PVdF-HFP matrix. In the TGA analysis of pure PVdF-HFP and the PVdF-HFP/GO nanocomposite separator materials (Fig. 1D), the PVdF-HFP polymer started to lose mass at 420 °C but retained more than 50% of the total mass up to 550 °C. The PVdF-HFP/GO separator began to lose mass at a slightly higher 440 °C but retained more than 50% at 550 °C.
We analyzed pore size distribution of the separators by using the mercury porosimetry to investigate the effect of adding the GO in PVdF-HFP separator. Through this measurement, we found that PVdF-HFP/GO separator could have more porosity, where the pore size diameter was between 1 μm and 100 μm, compared to pure PVdF-HFP separator (Fig. 2A and B). From this result we determined that increased porosity from 62.0% to 72.2 in the PVdF-HFP/GO separator can contribute to additional ion pathways for Li-ion transport. This effect is originated from the existence of GO in PVdF-HFP/GO separator.
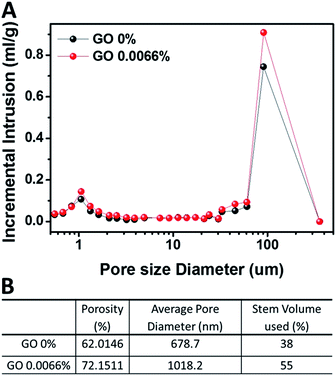 | ||
| Fig. 2 (A) Pore size distribution and (B) porosity and average pore diameter by measuring the mercury porosimetry of PVdF-HFP separator and PVdF-HFP/GO separator. | ||
The ionic conductivity of the GO nanosheets added PVdF-HFP separator was evaluated, as shown in Fig. 3A. As the GO nanosheets mass fraction increased to 0.0132 wt%, the ionic conductivity (σ) of the nanocomposite PVdF-HFP separator increased to 1.115 × 10−3 from 1.000 × 10−3 S cm−1. However the improvement of ionic conductivity was negligible. With an increasing content of GO nanosheets, the tensile strength of the PVdF-HFP separator also improved from 26.75 to 34.59 MPa (Fig. 3B) even though the increase of porosity after adding GO in PVdF-HFP. The improved tensile strength were directly related to the increase of the interfacial contact area between the GO nanosheets and PVdF-HFP polymer chains. In the conventional nanocomposite concept, flexible polymer chains enwrap the inserted GO nanosheets, which can improve the mechanical strength of the composite separators. The capacities were at a charge current of 0.2C and different discharged currents ranging from 0.2 to 3C (Fig. 3C). For all of the cells, a PVdF-HFP/GO nanocomposite separator showed the higher discharge capacities in overall discharge current densities than that of pure PVdF-HFP separator. As increasing GO contents in the separator up to 0.0044 wt%, the C-rate performances improved gradually, but when more than 0.0132 wt% of GO was added to the PVdF-HFP separator, aggregated GO spots were observed on the separator surface, which might give negative impact on the discharge capacities as shown in Fig. 3C. The capacities of the PVdF-HFP separators with GO nanosheets content (0.0033 to 0.0044 wt%) showed superior cell property to that of the pure PVdF-HFP separator. From this result, we can confirm that additional ionic pathways from GO nanosheets improved the transportation of Li-ion, thereby, overall capacities of PVdF-HFP/GO separator was enhanced.
 | ||
| Fig. 3 (A) Ionic conductivity at 25 °C. (B) Tensile strength. (C) Capacities of the cell at various discharge rates after charging at 0.2C for the separators with various contents of GO. | ||
Notwithstanding the PVdF-HFP separators having excellent dimensional stability (Fig. S4†), the state of the porous structure was intensively influenced by the existence of GO in the PVdF-HFP film. To confirm the effectiveness of introducing exfoliated GO nanosheets into the PVdF-HFP film, a pure PVdF-HFP separator and PVdF-HFP/GO nanocomposite separator were annealed at 150 and 170 °C, respectively, for 1 h (Fig. 4). Before annealing, the two separators had nearly identical pore structure. The pure PVdF-HFP separator completely melted and blocked its porous structure at 170 °C. On the other hand, the PVdF-HFP/GO separator maintained its pore shapes, suggesting the enhanced thermal and dimensional stability of the PVdF-HFP separator with added exfoliated GO.
 | ||
| Fig. 4 SEM micrographs of the PVdF-HFP/exfoliated GO nanosheets separator before and after being stored at 150/170 °C for 1 h. | ||
To determine the effect of the thermal stability on cell performances, the PVdF-HFP separators with exfoliated GO annealed at 150 and 180 °C were electrochemically examined by charging at 0.2C and discharging by increasing the current rate from 0.2 to 3C (Fig. 5). In case of the PVdF-HFP/GO nanocomposite separator annealed at 150 °C for 1 h, the discharging capacity maintains the original values of no heat treatment. However, the discharge capacity of the pure PVdF-HFP separator was nearly zero at 3C discharging condition. Surprisingly, the discharging capacity drop-rate of the 0.0066 wt% PVdF-HFP separator with exfoliated GO annealed at 180 °C for 1 h was also similar to that of no heat treatment.
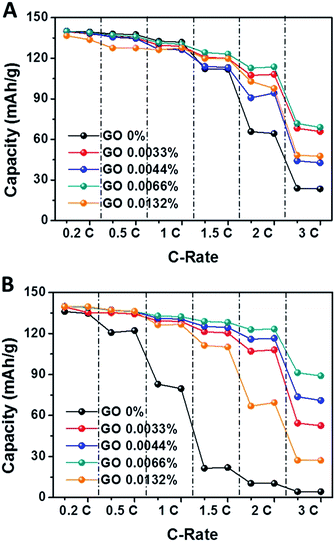 | ||
| Fig. 5 Discharge capacities of nanocomposite separators with various contents of exfoliated GO nanosheets after charging at 0.2C: (A) after being stored at 150 °C for 1 h and (B) 180 °C for 1 h. | ||
Conclusions
The addition of exfoliated graphene oxide nanosheets in the PVdF-HFP domain improved the electrochemical and mechanical properties and thermal stability of Li-ion battery separators. Due to the simple phase inversion method, this proposed process is cost-effective compared to the fabrication process of conventional PE separators. By means of dispersion of significantly exfoliated GO nanosheets within the PVdF-HFP film, the C-rate performance and thermal stability of Li-ion batteries were greatly enhanced. Therefore, the proposed separator might be a good alternative to PE separators for future battery technology.Acknowledgements
This work was supported by the Energy Efficiency & Resources Core Technology Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (20152010103470). This work was partially supported by the KIST Institutional Program (Project No. 2E25630). D. H. Kim thanks to National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2015M2A2A6A01045277).Notes and references
- H. Lee, M. Yanilmaz, O. Toprakci, K. Fu and X. Zhang, Energy Environ. Sci., 2014, 7, 3857 CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- X. Huang, J. Solid State Electrochem., 2011, 15, 649 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- G. Venugopal, J. Moore, J. Howard and S. Pendalwar, J. Power Sources, 1999, 77, 34 CrossRef CAS.
- Y. B. Kim, T. P. Thanh, M. Kim, D. W. Jung, G. R. Yi and J. H. Park, ACS Appl. Mater. Interfaces, 2015, 7, 4511 CAS.
- H. Zhang, M. Y. Zhou, C. E. Lin and B. K. Zhu, RSC Adv., 2015, 5, 89848 RSC.
- S. S. Zhang, J. Power Sources, 2007, 164, 351 CrossRef CAS.
- J. Shang, Y. Zhang, L. Yu, B. Shen, F. Lv and P. K. Chu, Mater. Chem. Phys., 2012, 134, 867 CrossRef CAS.
- P. H. Cury Camargo, K. G. Satyanarayana and F. Wypych, Mater. Res., 2009, 12, 1 CrossRef.
- M. A. Rahman and G. S. Chung, J. Alloys Compd., 2013, 581, 724 CrossRef.
- C. Da, F. Hongbin and L. Jinghong, Chem. Rev., 2012, 112, 6027 CrossRef PubMed.
- G. Vasilios, N. T. Jitendra, K. K. Christian, A. P. Jason, B. B. Athanasios, S. K. Kwang and Z. Radek, Chem. Rev., 2016, 116, 5464 CrossRef PubMed.
- E. Goki and C. Manish, Adv. Mater., 2010, 22, 2392 CrossRef PubMed.
- H. Jia-Qi, Z. Ting-Zhou, Z. Qiang, P. Hong-Jie, C. Chen-Meng and W. Fei, ACS Nano, 2015, 9, 3002 CrossRef PubMed.
- L. Kwan Ju and C. Young Hwan, Vacuum, 2014, 107, 269 CrossRef.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- L. J. Cote, F. Kim and J. Huang, J. Am. Chem. Soc., 2009, 131, 1043 CrossRef CAS PubMed.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. Huang, J. Am. Chem. Soc., 2010, 132, 8180 CrossRef CAS PubMed.
- M. Kim, J. K. Kim and J. H. Park, Adv. Funct. Mater., 2015, 25, 3399 CrossRef CAS.
- Y. Kojima, A. Usuki, M. Kawasumi, A. Okada, Y. Fukushima, T. Kurauchi and O. Kamigaito, J. Mater. Res., 1993, 8, 1185 CrossRef CAS.
- Y. Kojima, A. Usuki, M. Kawasumi, A. Okada, T. Kurauchi and O. Kamigaito, J. Appl. Polym. Sci., 1993, 49, 1259 CrossRef CAS.
- P. C. LeBaron, Z. Wang and T. J. Pinnavaia, Appl. Clay Sci., 1999, 15, 11 CrossRef CAS.
- K. K. Bissada, W. D. Johns and F. S. Cheng, Clay Miner., 1967, 7, 155 CAS.
- M. J. Koh, H. Y. Hwang, D. J. Kim, H. J. Kim, Y. T. Hong and S. Y. Nam, J. Mater. Sci. Technol., 2010, 26, 633 CAS.
- F. F. Azhar, A. Olad and A. Mirmohseni, Polym. Bull., 2014, 71, 1591 CrossRef.
- L. Priya and J. P. Jog, J. Polym. Sci. B Polym. Phys., 2003, 41, 31 CrossRef CAS.
- K. P. Pramoda, A. Mohamed, I. Y. Phang and T. X. Liu, Polym. Int., 2005, 54, 226 CrossRef CAS.
- E. P. Giannelis, Adv. Mater., 1996, 8, 29 CrossRef CAS.
- J. Wang, Z. Y. Liu, C. Y. Guo, Y. J. Chen and D. Wang, Macromol. Rapid Commun., 2001, 22, 1422 CrossRef CAS.
- K. Yano, A. Usuki, A. Okada, T. Kurauch and O. Kamigaito, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 2493 CrossRef CAS.
- D. R. Paul and L. M. Robeson, Polymer, 2008, 49, 3187 CrossRef CAS.
- J. W. Gilman, Appl. Clay Sci., 1999, 15, 31 CrossRef CAS.
- K. Norrish, Trans. Faraday Soc., 1954, 18, 120 RSC.
- M. Morvan, D. Espinat, J. Lambard and T. Zemb, Colloids Surf., A, 1994, 82, 193 CrossRef CAS.
- T. Rieker, A. Hanprasopwattana, A. Datye and P. Hubbard, Langmuir, 1999, 15, 638 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15062d |
| This journal is © The Royal Society of Chemistry 2016 |