DOI:
10.1039/C6RA15002K
(Paper)
RSC Adv., 2016,
6, 74845-74858
Synthesis of spiro isoindolinone-indolines and 1,2-disubstituted indoles from 2-iodobenzamide derivatives†
Received
9th June 2016
, Accepted 20th July 2016
First published on
22nd July 2016
Abstract
Copper catalyzed C-terminal and N-terminal attack of 2-alkynylanilines to 2-iodobenzamides afforded isoindolinones and 1,2-disubstituted indoles, respectively. Further, the isoindolinone derivatives were converted to spiro fused isoindolinone-indolines utilizing a halonium ion mediated strategy using NBS/TCC in the presence of acetic anhydride. Also, the ortho effect of the amide group in 2-iodobenzamide has been successfully applied for the synthesis of 1,2-disubstituted indoles.
Introduction
Spiroindoline derivatives have gained huge synthetic significance over the past few decades due to their biological activities and wide existence in several natural products.1 Particularly, the fused heterocycles spiroindolines have been known to exhibit a wide variety of biological activities including antiparasitic, antiviral, antitumor, and antibiotic agents, inhibitors of the human NK-1 receptor, and the gonadotropin-releasing hormone receptor antagonist.2 Further, spiroindoline derivatives also exhibit potential activity in controlling pests.3 Furthermore, fused isoindolinone spiro heterocyclic scaffolds are found as a core structure in a large family of bioactive products (Fig. 1).4
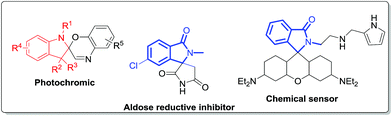 |
| | Fig. 1 Spiroindoline derivative and isoindolo-spiro hetero cyclic scaffolds. | |
Plethora methods were available for the synthesis of various heterocyclic fused spiroindolines in the literature.5 Moreover, to our knowledge, spiro fused isoindolinone-indoline frameworks were not reported. However, the similar structural analogs such as isoindolinone fused oxoindole frame works were reported (Scheme 1).6 The unique structural features and the diverse biological activity of core structures of both isoindolinone and indoline paved us the way to pay interest for synthesizing spiro fused structures of isoindolinone and indoline using readily available starting materials.
 |
| | Scheme 1 Previous reports on the synthesis of spiro-fused isoindolinone-indolinone derivatives. | |
On the other hand, 1,2-disubstituted indoles are also an important class of heterocycles because of their biological activities and medicinal applications including COX-2 inhibitors, estrogen receptor ligands, potential in the treatment of alzheimer's disease, in treatment of diseases associated with defects in vesicular (axonal) transport, treatment of inflammation and potent inhibitors of larval settlement by the barnacle, balanusamphitrite.7 A wide variety of procedures reported for the synthesis of 1,2-disubstituted indole derivatives.8 Among them cycloisomerization of 2-alkynylanilines9 is one of most frequently reported method for accessing 1,2-disubstituted indole derivatives. However, most of the reported procedures employed an expensive and air sensitive catalysts such as Pd, Au for this tranformation.10 Recently, Gao and co-workers had reported the synthesis of 1,2-disubstituted indole derivatives from aryl boronic acid and 2-alkynylanilines in the presence of copper catalyst and ligands.11
We12 and also others13 have explored the utility of 2-iodobenzamide derivatives for the synthesis of various bioactive N-heterocyclic compounds for the past few years. From our experience, and the literature14 available on the reaction of 2-iodobenzamide substrates under copper catalysis, we anticipated to prepare spiroisoindilinone-indoline derivatives as well as 1,2-disubstituted indole derivatives from 2-iodobenzamide derivatives as shown in proposed route (Scheme 2). We proposed that the treatment of 2-trimethylsilylethynylaniline 2a with 2-iodo-N-methylbenzamide 3a in the presence of copper catalyst may lead to form isoindolinone derivative via C-terminal attack (4a). Further, we presume that the treatment of 4a with a bromonium ion source may afford the corresponding bromospiroisoindolinone-indoline derivative 6aa. Moreover, we also expected that the replacement of 2-ethynylaniline with 2-(phenylethynyl)aniline in the present reaction, may form N-arylated compound 8aa via N-terminal attack which may further undergo cycloisomerization to furnish the corresponding 1,2-disubstituted indole 8a derivatives (Scheme 3).
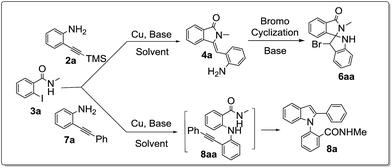 |
| | Scheme 2 Proposed routes for the spiroisoindolinone-indolines and 1,2-disubstituted indoles from 2-iodobenzamide derivatives. | |
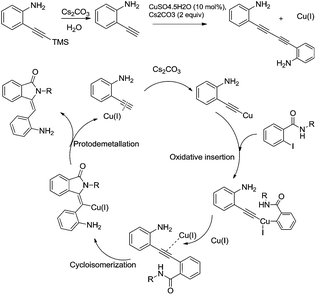 |
| | Scheme 3 Mechanism for the synthesis of 2-aminophenylisoindolinone derivatives. | |
Results and discussion
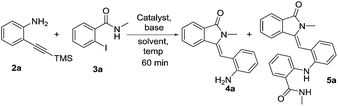
To test our assumption, we treated 2-(trimethylsilylethynyl)aniline 2a (1.0 mmol) with 2-iodo-N-methylbenzamide 3a (1.3 mmol) in the presence of potassium carbonate (2.0 equiv.), DMSO and copper iodide (20 mol%) at 100 °C for 1.5 h (entry 1, Table 1). Under these conditions, the expected compound 4a was obtained as a minor product along with unknown major product. Both these compounds were characterized using 1H NMR, 13C NMR and the mass spectra analysis. From the spectral data and X-ray analysis, the structure of the major product was found as 5a. On the other hand, when the internal 2-alkynyl aniline such as 2-(phenylethynyl)aniline 7a was used as coupling partner instead of trimethylsilyl 2-alkynylaniline 2a, we obtained the expected 1,2-disubstituted indole derivative 8a along with the intermediate N-arylated compound 8aa in the presence of potassium carbonate (3.0 equiv.), copper iodide (10 mol%) in DMSO solvent at 100 °C for 12 h (entry-1, Table 5).
Table 1 Optimization of the reaction for the formation of 3-(2-aminobenzylidene)-2-methylisoindolin-1-one 4a
|
|
| Entrya |
Catalyst |
Base |
Solvent |
2a (eq.) |
3a (eq.) |
Yieldb (%) |
|
4a
|
5a
|
|
All the reactions were carried out in 0.5 mmol scale using 2.0 equiv. of base in the indicated solvent (3.0 mL).
NMR yields.
|
| 1 |
Cul (20 mol%) |
K2CO3 |
DMSO |
1.0 |
1.3 |
15 |
55 |
| 2 |
Cul (20 mol%) |
K2CO3 |
DMSO |
1.2 |
1.0 |
62 |
21 |
| 3 |
Cul (20 mol%) |
K2CO3 |
DMSO |
1.3 |
1.0 |
80 |
9 |
| 4 |
Cul (20 mol%) |
Cs2CO3 |
DMSO |
1.3 |
1.0 |
48 |
34 |
| 5 |
Cul (20 mol%) |
K2CO3 |
DMF |
1.3 |
1.0 |
73 |
17 |
| 6 |
Cul (20 mol%) |
K2CO3 |
IPA |
1.3 |
1.0 |
40 |
18 |
| 7 |
Cu(OAc)2 (20 mol%) |
K2CO3 |
DMSO |
1.3 |
1.0 |
32 |
20 |
| 8 |
Cu(OAc)2 (20 mol%) |
Cs2CO3 |
DMF |
1.3 |
1.0 |
76 |
12 |
| 9 |
CuSO4·5H2O (20 mol%) |
Cs2CO3 |
DMF |
1.3 |
1.0 |
87 |
10 |
| 10 |
CuSO4·5H2O (10 mol%) |
Cs2CO3 |
DMF |
1.3 |
1.0 |
88 |
10 |
| 11 |
CuSO4·5H2O (5 mol%) |
Cs2CO3 |
DMF |
1.3 |
1.0 |
72 |
8 |
| 12 |
CuCl2 (10 mol%) |
Cs2CO3 |
DMF |
1.3 |
1.0 |
87 |
12 |
Encouraged by these initial results, we decided to optimize the reaction conditions for the synthesis of both isoindolinone derivatives and 1,2-disubstituted indole derivatives. Firstly, to optimize the reaction condition for the formation of isoindolinone derivatives, we examined the reaction to suppress the formation of undesired 5a (Table 1, entries 1–3). The reaction in the presence of 1.3 equiv. of 2a with respect to 1.0 equiv. of trimethylsilyl 2-alkynylaniline 3a, produced better result as compared to other conditions (entry 3). Further, increasing the basicity by replacing K2CO3 with Cs2CO3 leads to fall in yield. Polar solvents other than DMSO like DMF, IPA were also examined for this reaction (entries 5 and 6). We found that, DMF solvent is an alternate option for this reaction (entry 5). When we performed reaction in presence of copper(II) catalyst, satisfactory result was obtained. Unlike Cu(I), Cu(II) even using strong base Cs2CO3 afforded good yields (entries 7–12). However, with 10 mol% of CuSO4·5H2O, the reaction was very smooth with best yield (entry 10). From these optimization studies, it was concluded that the reaction in the presence of 10 mol% CuSO4·5H2O and 2.0 equiv. of base in DMF at 100 °C gave the best result.
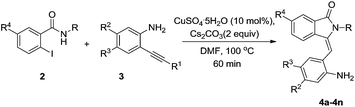
Having these optimized conditions in hand, we turned our attention to explore the reaction scope of this method. As shown in Table 2, various 2-alkynyl-phenylamines were reacted with 2-iodobenzamides, furnished 3-(2-aminobenzylidene)-2-substituted isoindolin-1-one derivatives. The reactions of 2-(phenylethynyl)aniline or 2-((trimethylsilyl)ethynyl)aniline with 2-iodo-N-methylbenzamide not shown much difference in the yields (Table 2, entries 1 and 2). When we conducted the reaction with 2-iodo-aliphatic N-substituted benzamides, with chloro and bromo substituents at R3 and R2 positions of substrate 3 produces good yields of desired product (entries 3–7). Subsequently, we tested the reactions with phenyl substituted 2-iodo-benzamide with substrate 3 produces the corresponding compounds 4g–4k in moderate to good yields (entries 8–12).
Table 2 Scope of the reactions for the synthesis of 3-(2-aminobenzylidene)-2-substituted isoindolin-1-one derivatives with respect to 2-iodobenzamides and 2-akynylanilinesa
|
|
| Entrya |
R |
R1 |
R2 |
R3 |
R4 |
Product |
Yieldb,c (%) |
|
Reaction conditions: 2 (1 mmol), 3 (1.2 equiv.), Cs2CO3 (2.0 equiv.), CuI (10 mol%), DMF (3.0 mL) at 100 °C.
Isolated yields.
In all the entries about 5–10% of undesired 5a analogs were observed.
|
| 1 |
Me |
H |
H |
H |
H |
4a
|
88 |
| 2 |
Me |
TMS |
H |
H |
H |
4a
|
86 |
| 3 |
Me |
TMS |
Br |
H |
H |
4b
|
83 |
| 4 |
Me |
TMS |
H |
Cl |
H |
4c
|
84 |
| 5 |
Ethyl |
TMS |
H |
Cl |
H |
4d
|
84 |
| 6 |
Allyl |
TMS |
H |
Cl |
H |
4e
|
85 |
| 7 |
–CH2CH2OMe |
TMS |
H |
Cl |
H |
4f
|
84 |
| 8 |
Ph |
TMS |
H |
H |
H |
4g
|
72 |
| 9 |
Ph |
TMS |
Br |
H |
Br |
4h
|
65 |
| 10 |
Ph |
TMS |
H |
Cl |
Br |
4i
|
68 |
| 11 |
Ph |
TMS |
OMe |
H |
H |
4j
|
82 |
| 12 |
Ph |
TMS |
H |
Cl |
H |
4k
|
71 |
| 13 |
–CH2Ph |
TMS |
H |
H |
H |
4l
|
72 |
| 14 |
–CH2Ph |
TMS |
Br |
H |
H |
4m
|
72 |
| 15 |
–CH2Ph |
TMS |
H |
Cl |
Br |
4n
|
67 |
Under optimized conditions, bromo substituted N-phenyl-2-iodobenzamides provided comparatively lower yields of the desired compounds (entries 9 and 10). Whereas in the presence of electron releasing group (OMe) on the 2-((trimethylsilyl)ethynyl)aniline resulted higher yield of the product (entry 11). Furthermore, N-benzyl-2-iodo-benzamide substrates were treated with substrate 3 under present conditions (entries 12–15). The proposed mechanism for the formation of isoindolinone derivatives from 2-iodobenzamides (3) and 2-akynylanilines was shown in Scheme 3.
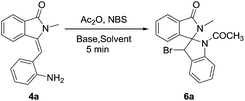
After synthesizing various structurally diverse 3-(2-aminobenzylidene)-2-substituted isoindolin-1-one derivatives, we focused our attention on the synthesis of spiro fused isoindolinone-indoline derivatives via C–N coupling. To determine suitable conditions for this reaction, we initially treated compound 4a with the combination of K2CO3 and NBS in the presence of acetonitrile at 25 °C and found multiple compounds which are complicate to isolate (Table 3). Then we presumed that in situ N-acetylation of compound 4a may favor the reaction to construct spiro fused isoindolinone-indoline derivatives. To determine reaction conditions for this reaction, we again treated compound 4a with a combination of acetic anhydride, K2CO3 and NBS sequentially, in the presence of acetonitrile at 40 °C. Under these conditions, the diastereomeric 1-acetyl-3-bromo-2′-methyl-1,3-dihydrospiro[indene-2,1′-isoindolin]-3′-one 6a product was obtained in 45% yield. The yield of the compound 6a improved to 62% by increasing the amount of acetic anhydride and NBS. When we conducted the reaction in the presence of 2.0 equiv. of Ac2O without solvent, the desired compound was obtained in 84% with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diastereoselectivity ratio.
2 diastereoselectivity ratio.
Table 3 Optimization of reaction for the formation of 1-acetyl-3-bromo-2′-methyl-1,3-dihydrospiro[indene-2,1′-isoindolin]-3′-one 6a
Still, to increase the diastereoselectivity, reaction was carried out at 0 °C favoring the kinetically control rather than thermodynamic control, which results drastic fall in yield which may be due to the less driving force for the reaction to proceed forward. Furthermore, at 25 °C, satisfactory results were obtained in both yields and diastereoselectivity. The excess addition of acetic anhydride in the reaction did not show any marked improvement in terms of yields and diastereoselectivity. Usage of strong base also shows the fall in yields resulting a black charred reaction mixture which is due to the decomposition of product at highly basic condition. From these optimization studies, it was concluded that the reaction in the presence of 2.0 equiv. of Ac2O, 3.0 equiv. of K2CO3 and 1.2 equiv. of NBS at 25 °C gave the best result in terms of product yields and diastereoselectivity.
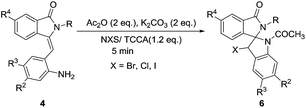
Having this optimized reaction condition in hand, we next employed the synthesis of various structurally diverse 3-(2-aminobenzylidene)-2-substituted isoindolin-1-one derivatives (Table 4). In this regard, first, we conducted the reaction using methyl and ethyl substituted isoindolin-1-one derivatives 4a–4d. Under this optimized condition, these substrates produced the inseparable diastereomers of spiro fused isoindolinone-indolines with good yields and diastereoselectivity (Table 4, entries 1–4). Further, we tested the reactivity of compound 4f using different halogen sources like NBS, NCS, and NIS. The reaction in presence of NBS resulted the good yields of product with single diastereomer (entry-5). Whereas, in the case of NCS reaction was not facilitated. However, trichloroisocyanuric acid (TCCA) gave expected compound in good yield and diastereoselectivity (entry 6). The reaction in the presence of NIS resulted decomposition of the reaction (entry 7). 3-(2-Aminobenzylidene)-2-benzylisoindolin-1-one derivatives 4l–4n provided the desired inseparable diastereomeric products 6h–6j in 71–78% yield (entries 8–10). Whereas, phenyl substituted-isoindolin-1-one derivatives 4g–4i and 4k furnished these inseparable diastereomers of spiro fused isoindolinoneindolines 6k–6n in moderate yields (entries 11–14). This may be due to the restricted environment of bulky benzyl or phenyl groups, respectively, for spirocyclizations. The crystal structures of both the diastereomers of 1-acetyl-3-bromo-5-chloro-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one 6n and 6n′ were shown in Fig. 2. The reaction with methoxy substituted derivative 4j resulted the unexpected product in which the halogen was replaced by OCOCH3 group with good yield and diastereoselectivity (entry 15). Allyl substituted-isoindolin-1-one derivative 4e, undergoes cyclo propyl bromonium ion intermediate formation predominantly to internal double bond than external double bond to afford desired spiro compound (entry 16).
Table 4 Scope of the reactions for the synthesis of 1-acetyl-3-bromo-2′-methyl-1,3-dihydrospiro[indene-2,1′-isoindolin]-3′-one derivatives (6)a
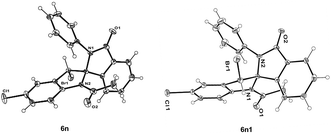 |
| | Fig. 2 Crystal structures of 1-acetyl-3-bromo-5-chloro-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one 6n and 6n1 diastereomers. | |
In order to check the feasibility for scale up, we synthesized compound 4f from its corresponding precursors in 15.0 mmol scale with 88% isolated yield, which is further converted to spiro fused isoindolinone-indoline 6e with 82% isolated yield and single diastereomer (Scheme 4).
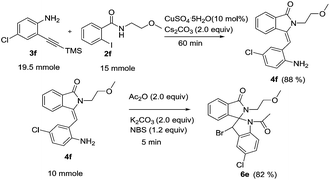 |
| | Scheme 4 Scale up syntheses of compounds 4f and 6e. | |
Mechanism for the formation of 1-acetyl-3-bromo-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one derivatives could be depicted in Scheme 5.
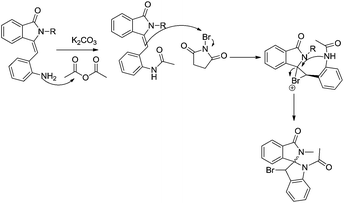 |
| | Scheme 5 Plausible mechanism for formation spiro[indoline-2,1′-isoindolin]-3′-one derivatives. | |
On the other hand, synthesis of N-benzyl-2-(2-phenyl-1H-indol-1-yl)benzamide 8a was investigated for its optimization as shown in Table 5. Initial screening of this reaction was started with 10 mol% of CuI with 1.2 equiv. of 7a, 1.0 equiv. of 3a, and 3.0 equiv. of K2CO3 in DMSO. The yield is very low resulting the decomposition of 3a and unreacted 7a was recovered (Table 5, entry 1). Then the catalyst loading was increased to 20 mol% and checked the reactivity with 1.3 equiv. and 1.5 equiv. of 3a and it was found that there is an improvement in yield (entries 2 and 3). Subsequently the yield was still improved by altering the base from K2CO3 to Cs2CO3 (entry 4). But, there is no further improvement in the yield, even the addition of excess of 3a (entry 5). The other sources of copper like CuBr and CuSO4 were also examined and found not satisfactory (entries 6–8). The usage of ligands leads to the decomposition of 3a, no further reaction and 7a was recovered (entries 9 and 10). Other kinds of polar solvents also examined which were not satisfactory (entries 11–14).
Table 5 Optimization of reaction for the formation of N-benzyl-2-(2-phenyl-1H-indol-1-yl)benzamide 8a
|
|
| Entrya |
Catalyst (mol%) |
Ligand |
t (h) |
7a (eq.) |
3a (eq.) |
Base (3 eq.) |
Solvent |
Productc (%) |
|
All the reactions were performed in 0.5 mmol scale with 7a (0.5 mmol), at 100 °C and 20 mol% of catalyst.
7a was not consumed completely. Unreacted 7a was recovered.
NMR yields. 1,10-Phen = 1,10-phenanthraline.
10 mol% of catalyst used.
|
| 1 |
Cul |
— |
12 |
1.2 |
1 |
K2CO3 |
DMSO |
22b,d |
| 2 |
Cul |
— |
12 |
1 |
1.3 |
K2CO3 |
DMSO |
45b |
| 3 |
Cul |
— |
12 |
1 |
1.5 |
K2CO3 |
DMSO |
50b |
| 4 |
Cul |
— |
7 |
1 |
1.5 |
Cs2CO3 |
DMSO |
71 |
| 5 |
Cul |
— |
7 |
1 |
1.7 |
Cs2CO3 |
DMSO |
71 |
| 6 |
Cul(OAc)2 |
— |
12 |
1 |
1.5 |
Cs2CO3 |
DMSO |
10b |
| 7 |
CuBr |
— |
12 |
1 |
1.5 |
Cs2CO3 |
DMSO |
30b |
| 8 |
CuSO4 |
— |
12 |
1 |
1.5 |
Cs2CO3 |
DMSO |
—b |
| 9 |
Cul |
L-Proline |
12 |
1 |
1.5 |
Cs2CO3 |
DMSO |
46b |
| 10 |
Cul |
1,10-Phen |
12 |
1 |
1.5 |
Cs2CO3 |
DMSO |
— |
| 11 |
Cul |
— |
9 |
1 |
1.5 |
Cs2CO3 |
DMF |
65b |
| 12 |
Cul |
— |
12 |
1 |
1.5 |
Cs2CO3 |
THF |
48b |
| 13 |
Cul |
— |
12 |
1 |
1.5 |
Cs2CO3 |
IPA |
—b |
| 14 |
Cul |
— |
12 |
1 |
1.5 |
Cs2CO3 |
1,4-Dioxane |
—b |
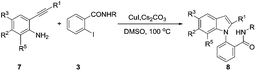
Having the optimized conditions in hand, the scope of the above reaction was also examined as showed in Table 6. The reactivity of different kinds of N-substituted 2-iodo benzamides were studied (Table 6, entries 1–11) and N-substituted 3-OMe propyl group at R gives the maximum yield (entry-9). The reactivity was also checked by taking different aliphatic substituents at R1 which afforded low or moderate yields (entries 12–14). However, presence of electron releasing functional group at R1 results good yield (entry 15). The presence of electron releasing groups at R2 position gives moderate yields (entries 16 and 17). But, the presence of electron withdrawing group –NO2 in the same position gives good yield (entry 18). With chloro substituents at R3 position gives moderate yields (entries 19 and 20). The presence of aliphatic chain at R1, the yield is expected to be low. But, may be due to effect of N-substituted 3-methoxy amidic group, even in the presence of aliphatic group at R1, the yield is reasonably good (entry 21).
Table 6 Scope of the reactions for the synthesis of 1,2-substituted indolyl N-substituted benzamide derivatives (8) with respect to 2-iodobenzamides (3) and 2-akynylanilines (7)
|
|
| Entrya |
R |
R1 |
R3 |
R2 |
R5 |
t (h) |
Product |
Yieldb (%) |
|
Reaction conditions: 7 (1 mmol), 3 (1.5 mmol), Cs2CO3 (2 mmol), CuI (20 mol%), DMSO (3 mL).
Isolated yields.
Reaction is incomplete and the starting material was recovered.
|
| 1 |
Me |
Ph |
H |
H |
H |
6 |
8a
|
47 |
| 2 |
Ph |
Ph |
H |
H |
H |
4 |
8b
|
59 |
| 3 |
o-Tolyl |
Ph |
H |
H |
H |
4 |
8c
|
66 |
| 4 |
p-OMe-phenyl |
Ph |
H |
H |
H |
4 |
8d
|
68 |
| 5 |
Benzyl |
Ph |
H |
H |
H |
7 |
8e
|
71 |
| 6 |
CH2CH(Ph)2 |
Ph |
H |
H |
H |
4 |
8f
|
73 |
| 7 |
Ethyl |
Ph |
H |
H |
H |
4 |
8g
|
68 |
| 8 |
Allyl |
Ph |
H |
H |
H |
4 |
8h
|
75 |
| 9 |
(CH2)3–OMe |
Ph |
H |
H |
H |
4 |
8i
|
79 |
| 10 |
CH2–CH2-Ph |
Ph |
H |
H |
H |
4 |
8j
|
77 |
| 11 |
(CH2)4Ph |
Ph |
H |
H |
H |
4 |
8k
|
74 |
| 12 |
Benzyl |
Cyclopentyl |
H |
Cl |
H |
18 |
8l
|
48 |
| 13 |
Me |
Cyclohexyl |
H |
Me |
H |
19 |
8m
|
49 |
| 14 |
Benzyl |
Isopropyl |
Me |
H |
Me |
18 |
8n
|
51 |
| 15 |
Benzyl |
–CH2OMe |
OMe |
H |
H |
3 |
8o
|
70 |
| 16 |
Benzyl |
Ph |
H |
OMe |
H |
7 |
8p
|
63 |
| 17 |
Benzyl |
Ph |
H |
Me |
H |
8 |
8q
|
67 |
| 18 |
Benzyl |
Ph |
H |
NO2 |
H |
6 |
8r
|
76 |
| 19 |
Benzyl |
Ph |
Cl |
H |
H |
7 |
8s
|
55 |
| 20 |
Ph |
Ph |
Cl |
H |
H |
7 |
8t
|
61 |
| 21 |
(CH2)3–OMe |
Heptyl |
Cl |
H |
H |
12 |
8u
|
67 |
| 22 |
Benzyl |
4-Cl, 2-Br-phenyl |
Cl |
H |
H |
22 |
8v
|
39c |
| 23 |
(CH2)3–OMe |
4-Cl, 2-Br-phenyl |
Cl |
H |
H |
9 |
8w
|
64 |
The N-benzyl substituted-2-iodobenzamide is very less reactive and less stable in the presence of other partner substrate and hence decomposed and the other substrate 2-phenylalkynylaniline derivative was recovered (entry 22). Whereas, replacing with N-substituted-3-MeO propyl group at R resulted in completion of reaction with good yield (entry 23). Further, the proposed the mechanism for the formation of 2-arylamidoindole derivatives from 2-iodobenzamides (3) and 2-akynylanilines was shown in Scheme 6.
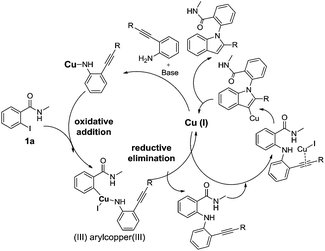 |
| | Scheme 6 Plausible mechanism for the synthesis of I, 2 disubstituted indole derivatives. | |
Next, to test the limitation of this methodology and to prove that ortho effect of N-substituted amidic group is playing a key role for its reactivity, we executed some experiments which are limitations for this protocol, on reacting various ortho substituted iodo benzenes I with 2-phenylalkynylaniline 7a and found no reaction even after 24 h as shown in Scheme 7.
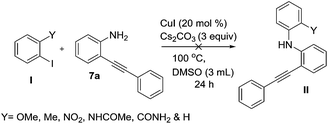 |
| | Scheme 7 Experiments to prove the ortho effect of N-substituted amidic group for its reactivity. | |
Finally, the indole substrate containing bromo group such as 1-(2-(2-(2-bromo-4-chlorophenyl)-5-chloro-1H-indol-1-yl)phenyl)-4-methoxybutan-1-one 8w was subjected for cyclization in presence of palladium acetate, triphenylphosphine and cesium carbonate in dioxane at 140 °C to synthesize 3,12-dichloro-N-(3-methoxypropyl) indolo[1,2-f]phenanthridine-8-carboxamide 9 (Scheme 8).
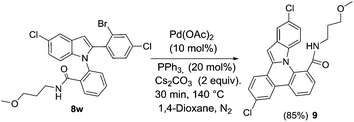 |
| | Scheme 8 Synthesis of N-substituted indolo[1,2-f]phenanthridinecarboxamide. | |
Conclusions
In conclusion, copper catalyzed synthesis of isoindolinones and 1,2-disubstituted indoles from a common starting material has been reported. We have developed an easy access for the synthesis of spiro fused isoindolinone-indolines utilizing a well-known, halogen sources mediated cyclopropylhalonium ion strategy. Very few methods are known in literature14h where TMS terminated alkynyl anilines participated in further transformation without TMS deprotected. Also, we successfully utilized the ortho-effect of amide group in N-substituted 2-iodobenzamides for the ligand free synthesis of 1,2-disubstituted indolylcarboxamides which also provide a path for the synthesis of indolophenanthradine carboxamide derivative.
General information
Reagents and solvents were purchased from various commercial sources and were used directly without any further purification, unless otherwise stated. Column chromatography was performed with 63–200 mesh silica gel. 1H and 13C NMR spectra were recorded at 400 and 100 MHz, respectively. Chemical shifts are reported in parts per million (δ) using chloroform as internal standards and coupling constants are expressed in Hertz. Melting points were recorded using an electro thermal capillary melting point apparatus and are uncorrected.
General procedure for the synthesis of 3-(2-aminobenzylidene)-2-substituted isoindolin-1-one derivatives (4a–4n)
Copper sulphate (10 mole%) and cesium carbonate (2.0 equiv.) were added to a stirred solution of 2-iodo-N-substituted benzamide (1 mmol), 2-((trimethylsilyl)alkynyl)aniline or 2-alkynylaniline 1.3 equiv. (1.3 mmol) in dimethylsulfoxide as a solvent under open air atmosphere. The reaction mixture was then heated to 100 °C. The reaction was monitored by TLC. After completion of the reaction, the resulting reaction mixture was cooled to room temperature and was then extracted with ethyl acetate, washed with brine and dried over MgSO4. The resulting crude compound was purified by flash column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) on silica gel to afford desired product.
4) on silica gel to afford desired product.
General procedure for the synthesis of 1,2 substituted indolyl N-substituted benzamide derivatives (8a–8w)
Copper iodide (20%) and cesium carbonate (3.0 equiv.) were added to a stirred solution of 2-(substituted alkynyl)aniline 1.0 equiv. (1.0 mmol), 2-iodo-N-substituted benzamide (1.5 mmol), in dimethylsulfoxide as a solvent under open air atmosphere. The reaction mixture was then heated to 100 °C. The reaction was monitored by TLC. After completion of the reaction the resulting reaction mixture was cooled to room temperature and was then extracted with ethyl acetate, washed with brine and dried over MgSO4. The resulting crude compound was purified by flash column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) on silica gel to afford desired product.
4) on silica gel to afford desired product.
General procedure for the synthesis of 1-acetyl-3-halo-2′-methyl-1,3-dihydrospiro[indene-2,1′-isoindolin]-3′-one derivatives (6a–6p)
To the stirred suspension of 3-(2-aminobenzylidene)-2-substituted isoindolin-1-one derivatives (4) (0.5 mmol) and acetic anhydride (2 equiv.), added potassium carbonate (3.0 equiv.) fallowed by N-bromosuccinimide (NBS)/trichloroisocyanuric acid (TCCA) (1 equiv.) at 25 °C. The reaction was monitored by TLC. After completion of the reaction the resulting reaction mixture was cooled to room temperature and was then extracted with ethyl acetate, washed with brine and dried over MgSO4. The resulting crude compound was purified by flash column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on silica gel to afford desired product.
1) on silica gel to afford desired product.
General procedure for the synthesis of 3,12-dichloro-N-(3-methoxypropyl) indolo[1,2-f]phenanthridine-8-carboxamide (9)
To 0.5 mmol of stirred suspension of compound 8w and 1,4 dioxane, added pd(OAc)2 10 mol%, pph3 20 mole%, and Cs2CO3 (2.0 equiv.) under nitrogen atmosphere, and heated the contents for 30 min at 140 °C. The reaction was monitored by TLC. After completion of the reaction the resulting reaction mixture was cooled to room temperature and was then extracted with ethyl acetate, washed with brine and dried over MgSO4. The resulting crude compound was purified by flash column chromatography (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) on silica gel to afford desired product.
3) on silica gel to afford desired product.
N-Methyl-2-(2-phenyl-1H-indol-1-yl)benzamide (8a).
170 mg, 47% yield; colorless solid, mp 153–155 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.94 (dd, J = 7.3, 1.7 Hz, 1H), 7.70–7.68 (m, 1H), 7.53–7.47 (m, 2H), 7.33 (J = 1.1 Hz, 1H), 7.24–7.18 (m, 5H), 7.09 (t, J = 4.6 Hz, 3H), 6.86 (s, 1H), 2.50 (d, J = 4.8 Hz, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 166.2, 141.5, 139.1, 135.9, 134.1, 131.8, 131.1, 130.1, 128.8, 128.6, 128.0, 123.2, 121.5, 121.0, 110.8, 104.3, 27.0. LRMS (EI+) (m/z) (relative intensity): 326.1 (M+, 100), 268.0 (92), 296.1 (30). HRMS (TOF-ES)+ calcd for C22H18N2O (M + Na): 349.1317, found 349.1320.
N-Phenyl-2-(2-phenyl-1H-indol-1-yl)benzamide (8b).
229 mg, 59% yield; colorless solid, mp 210–212 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.05 (d, J = 1.8 Hz, 1H), 7.74 (dd, J = 7.9, 1.5 Hz, 1H), 7.63 (t, J = 7.4 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.49 (d, J = 7.7 Hz, 1H), 7.27–7.12 (m, 10H), 6.98 (t, J = 7.3 Hz, 1H) 6.86 (t, J = 6.9 Hz, 3H), 6.78 (s, 1H). 13C NMR (100 MHz CDCl3) δC (ppm): 163.1, 141.8, 138.8, 137.7, 135.7, 134.3, 132.3, 131.8, 131.4, 130.3, 129.2, 128.8, 128.6, 128.2, 124.4, 123.6, 121.9, 121.2, 119.9, 110.7, 104.7. LRMS (EI+) (m/z) (relative intensity): 296.1 (100), 388.2 (M+, 93), 267.1 (44). HRMS (TOF-ES)+ calcd for C27H21N2O (M + H): 389.1654, found 389.1653.
2-Phenyl-1H-indol-1-yl)-N-(o-tolyl)benzamide (8c).
265 mg, 66% yield; colorless solid, mp 183–185 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.15–8.13 (m, 1H), 7.78 (d, J = 8.1 Hz, 1H), 7.67–7.65 (m, 1H), 7.59–7.51 (m, 2H), 7.30 (dd, J = 2.1, 8.4 Hz, 1H), 7.31–7.29 (m, 9H), 7.13–7.09 (m, 1H), 6.95–6.90 (m, 2H), 6.83 (s, 1H), 1.26 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 163.3, 141.8, 139.4, 135.9, 135.8, 133.9, 132.4, 131.9, 131.5, 130.3, 130.1, 129.1, 129.0, 128.7, 128.7, 128.1, 126.6, 124.7, 123.6, 122.0, 121.2, 111.0, 105.1, 16.3. LRMS (EI+) (m/z) (relative intensity): 296.1 (100), 402.2 (M+, 81), 267.1 (44). HRMS (TOF-ES)+ calcd for C28H23N2O (M + H): 403.1810, found 403.180.
N-(4-Methoxyphenyl)-2-(2-phenyl-1H-indol-1-yl)benzamide (8d).
284 mg, 68% yield; colorless solid, mp 170–173 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.04 (dd, J = 7.64, 1.34 Hz, 1H), 7.73 (dd, J = 6.96, 2.72 Hz, 1H), 7.62–7.58 (m, 1H), 7.55–7.51 (m, 1H), 7.46 (d, J = 7.72 Hz, 1H), 7.26–7.13 (m, 8H), 6.84 (s, 1H), 6.80 (d, J = 9.0, 2H), 6.70 (s, 1H), 6.76 (d, J = 8.9, 2H), 3.71 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 162.8, 156.4, 141.7, 138.8, 135.6, 134.3, 132.1, 131.7, 131.4, 130.9, 130.2, 129.1, 128.8, 128.7, 128.6, 128.1, 123.5, 121.9, 121.6, 121.2, 114.0, 110.7, 104.6, 55.5. LRMS (EI+) (m/z) (relative intensity): 296.1 (100), 418.2 (M+, 88), 267 (38). HRMS (TOF-ES)+ calcd for C28H23N2O2 (M + H): 419.1760, found 419.1760.
N-Benzyl-2-(2-phenyl-1H-indol-1-yl)benzamide (8e).
287 mg, 71% yield; colorless solid, mp 132–134 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.02 (dd, J = 7.5, 2.04 Hz, 1H), 7.65 (d, J = 7.3 Hz, 1H), 7.55–7.48 (m, 2H), 7.28 (dd, J = 7.3, 1.46 Hz, 1H), 7.21–7.10 (m, 9H), 7.03 (d, J = 7.9 Hz, 1H), 6.75 (s, 1H), 6.70 (d, J = 6.9 Hz, 2H), 5.54 (bs, 1H), 4.20–4.11 (m, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.3, 141.1, 139.2, 137.2, 135.9, 134.1, 131.9, 131.7, 131.3, 130.5, 128.9, 128.6, 128.6, 127.9, 127.8, 127.4, 123.3, 121.5, 121.1, 110.7, 104.5, 44.4. LRMS (EI+) (m/z) (relative intensity): 268.1 (100), 402.2 (M+, 71), 296.1 (38). HRMS(TOF-ES)+ calcd for C28H23N2O (M + H): 403.1810, found 403.1809.
N-(2,2-Diphenylethyl)-2-(2-phenyl-1H-indol-1-yl)benzamide (8f).
359 mg, 73% yield; colorless solid, mp 168–170 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.87 (dd, J = 7.26, 1.70 Hz, 1H), 7.72 (d, J = 7.80 Hz, 1H), 7.58–7.52 (m, 2H), 7.47–7.40 (m, 2H), 7.26–7.23 (m, 5H), 7.20–7.05 (m, 8H), 7.00 (d, J = 8.16 Hz, 1H), 6.91 (d, J = 8.16 Hz, 2H), 6.80 (d, J = 6.96, 2H), 6.76 (s, 1H), 5.37 (bs, 1H), 3.68 (m, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.5, 141.6, 141.5, 141.4, 139.2, 136.0, 133.8, 131.8, 131.7, 131.2, 130.0, 128.7, 128.6, 128.5, 128.5, 127.8, 127.8, 126.8, 123.3, 121.6, 121.0, 110.9, 104.7, 50.2, 44.4. LRMS (EI+) (m/z) (relative intensity): 296.1 (100), 492.2 (M+, 50), 267 (28). HRMS (TOF-ES)+ calcd for C35H29N2O (M + H): 493.2280, found 493.2278.
N-Ethyl-2-(2-phenyl-1H-indol-1-yl)benzamide (8g).
231 mg, 68% yield; colorless solid, mp 127–129 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.97 (dd, J = 3.8, 3.14 Hz, 1H), 7.68 (d, J = 4.4 Hz, 1H), 7.52–7.45 (m, 2H), 7.28 (t, J = 4.4 Hz, 1H), 7.25–7.20 (m, 5H), 7.17 (t, J = 3.5 Hz, 2H), 7.05 (t, J = 4.3 Hz, 1H), 6.86 (s, 1H), 5.14 (bs, 1H), 3.03–2.92 (m, 2H), 0.55 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm): 165.3, 141.1, 139.1, 135.6, 134.4, 131.7, 131.2, 130.2, 128.9, 128.6, 128.5, 128.4, 127.9, 123.2, 121.4, 120.9, 110.7, 104.2, 34.7, 13.9. LRMS (EI+) (m/z) (relative intensity): 268.0 (100), 340.2 (M+, 90). HRMS (TOF-ES)+ calcd for C23H21N2O (M + H): 341.1654, found 341.1653.
N-Allyl-2-(2-phenyl-1H-indol-1-yl)benzamide (8h).
264 mg, 75% yield; colorless solid, mp 119–121 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.97 (dd, J = 3.8, 3.2 Hz, 1H), 7.68 (d, J = 3.8 Hz, 1H), 7.76–7.48 (m, 2H), 7.28 (d, J = 4.3 Hz, 1H), 7.25–7.21 (m, 5H), 7.19–7.16 (m, 2H), 7.06 (t, J = 4.8 Hz, 1H), 6.86 (s, 1H), 5.28 (bs, 1H), 5.34–5.24 (m, 1H), 4.82 (d, J = 10.3 Hz, 1H), 4.68 (d, J = 17.2 Hz, 1H), 3.60–3.55 (m, 2H), 13C NMR (100 MHz CDCl3) δC (ppm): 165.4, 141.1, 139.2, 135.9, 133.2, 131.9, 131.7, 131.3, 128.9, 130.3, 128.9, 128.6, 128.6, 128.0, 123.3, 121.5, 121.0, 116.5, 110.7, 104.5, 42.5. LRMS (EI+) (m/z) (relative intensity): 268.0 (100), 296.1 (98), 352.0 (M+, 94). HRMS (TOF-ES)+ calcd for C24H21N2O (M + H): 353.1654, found 353.1651.
N-(3-Methoxypropyl)-2-(2-phenyl-1H-indol-1-yl)benzamide (8i).
274 mg, 79% yield; colorless solid, mp 126–128 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.02 (dd, J = 6.1, 3.3 Hz, 1H), 7.64 (d, J = 3.0 Hz, 1H), 7.50 (t, J = 3.9 Hz, 2H), 7.25 (dd, J = 6.6, 2.1 Hz, 2H), 7.21 (s, 1H), 7.17–7.14 (m, 3H), 7.05–7.00 (m, 3H), 6.82 (s, 1H), 5.27 (bs, 1H), 3.03–2.95 (m, 2H), 2.35 (d, J = 7.6 Hz, 2H), 1.15–1.09 (m, 2H), 0.98–0.92 (m, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.6, 141.2, 139.3, 135.8, 134.3, 131.8, 131.7, 131.2, 130.2, 128.7, 128.6, 127.9, 123.2, 121.5, 121.0, 110, 8, 104.4, 58.6, 37.9, 28.9. LRMS (EI+) (m/z) (relative intensity): 296.1 (100), 384.2 (M+, 92), 268(68). HRMS (TOF-ES)+ calcd for C25H25N2O2 (M + H): 385.1916, found 385.1917.
N-Phenethyl-2-(2-phenyl-1H-indol-1-yl)benzamide (8j).
320 mg, 77% yield; colorless solid, mp 135–137 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.93 (dd, J = 7.3, 1.5 Hz, 1H), 7.70 (dd, J = 6.5, 2.4 Hz, 1H), 7.50–7.46 (m, 2H), 7.26 (dd, J = 6.7 Hz, 2.32 Hz, 1H), 7.23–7.16 (m, 7H), 7.10–7.06 (m, 4H), 6.85 (s, 1H), 6.79 (dd, J = 6.9, 2.4 Hz, 2H), 5.29 (bs, 1H), 3.26–3.20 (m, 2H), 2.26 (t, J = 7.4 Hz, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.4, 142.1, 141.2, 139.2, 135.7, 134.3, 131.8, 131.7, 131.4, 130.3, 129.0, 128.6, 128.5, 128.5, 128.4, 128.0, 125.9, 123.3, 121.6, 121.0, 110.7, 104.4, 39.8, 35.5, 28.5, 28.2. LRMS (EI+) (m/z) (relative intensity): 296.1 (100), 416.0 (M+, 98), 312 (70). (M + H, 100), HRMS (TOF-ES)+ calcd for C29H25N2O (M + H): 417.1967, found 417.1965.
2-(2-Phenyl-1H-indol-1-yl)-N-(4-phenylbutyl)benzamide (8k).
329 mg, 74% yield; colorless solid, mp 113–115 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.01–8.04 (m, 1H), 7.68–7.72 (m, 1H), 7.50 (t, J = 4.2 Hz, 2H), 7.25 (dd, J = 6.6, 1.9 Hz, 2H), 7.21 (s, 5H), 7.17–7.14 (m, 3H), 7.05–7.00 (m, 3H), 6.82 (s, 1H), 3.04–2.93 (m, 2H), 2.35 (t, J = 7.5 Hz, 2H), 1.17–1.11 (m, 2H), 0.98–0.91 (m, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.4, 142.1, 141.2, 139.2, 135.7, 134.3, 131.8, 131.7, 131.4, 130.3, 129.0, 128.6, 128.5, 128.5, 128.4, 128.0, 125.9, 123.3, 121.6, 121.0, 110.7, 104, 42, 39.8, 35.5, 28.5, 28.2. LRMS (EI+) (m/z) (relative intensity): 444.2 (M+, 100), 268.1 (72), 296 (68). HRMS (TOF-ES)+ calcd for C31H29N2O (M + H): 445.2280, found 445.2280.
N-Benzyl-2-(6-chloro-2-cyclopentyl-1H-indol-1-yl)benzamide (8l).
205 mg, 48% yield; colorless solid, mp 115–117 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.15–8.13 (m, 1H), 7.62–7.59 (m, 2H), 7.44 (d, J = 2.0 Hz, 1H), 7.28 (dd, J = 5.2, 2.5 Hz, 1H), 7.19–7.11 (m, 3H), 6.97 (dd, J = 8.6, 2.1 Hz, 1H), 6.73 (d, J = 8.7 Hz, 1H), 6.64 (d, J = 6.7 Hz, 1H), 6.26 (s, 1H), 4.16–4.05 (m, 2H), 2.78–2.70 (m, 1H), 1.80–1.43 (m, 8H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.1, 148.4, 136.8, 136.5, 134.7, 134.6, 132.1, 131.6, 130.5, 129.6, 129.4, 128.6, 127.8, 127.5, 126.5, 122.3, 119.9, 110.7, 99.0, 44.5, 37.4, 34.0, 32.7, 25.4, 25.3. LRMS (EI+) (m/z) (relative intensity): 428.2 (M+, 100), 321.1 (24). HRMS (TOF-ES)+ calcd for C27H26N2OCl (M + H): 429.1734, found 429.1732.
2-(2-Cyclohexyl-6-methyl-1H-indol-1-yl)-N-methylbenzamide (8m).
169 mg, 49% yield; colorless solid, mp 139–141 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.18 (dd, 7.3, 2.0 Hz, 1H), 7.63–7.59 (m, 2H), 7.47 (d, J = 8.0 Hz, 1H), 7.32 (t, J = 4.4 Hz, 1H), 6.96 (d, J = 7.9 Hz, 1H), 6.68 (s, 1H), 6.41 (s, 1H), 4.96 (bs, 1H), 2.45 (d, J = 4.5 Hz, 3H), 2.36 (s, 3H), 1.81–1.49 (m, 7H), 1.26–1.11 (m, 4H). 13C NMR (100 MHz CDCl3) δC (ppm): 166.2, 147.4, 138.2, 135.2, 134.1, 132.1, 132.0, 131.8, 130.3, 129.2, 126.4, 122.7, 120.1, 110.0, 99.4, 35.7, 34.7, 32.5, 27.0, 26.5, 26.4, 26.1, 21.9. LRMS (EI+) (m/z) (relative intensity): 346.2 (M+, 100), 303.1 (22). HRMS (TOF-ES)+ calcd for C23H27N2O (M + H): 347.2123, found 347.2121.
N-Benzyl-2-(2-isopropyl-5,7-dimethyl-1H-indol-1-yl)benzamide (8n).
202 mg, 51% yield; colorless solid, mp 116–118 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.23 (dd, 7.6, 1.8 Hz, 1H), 7.61–7.52 (m, 2H), 7.34 (dd, J = 7.6, 1.6 Hz, 1H), 7.17–708 (m, 4H), 6.65 (t, J = 4.4 Hz, 3H), 6.25 (s, 1H), 5.56 (bs, 1H), 4.17–4.07 (m, 2H), 2.25–2.46 (m, 1H), 2.38 (s, 3H), 1.74 (s, 3H), 1.04 (dd, J = 15.4, 6.6 Hz, 6H). 13C NMR (100 MHz, CDCl3) δC (ppm): 164.9, 148.7, 137.3, 137.2, 134.7, 133.9, 131.6, 131.4, 131.3, 130.2, 129.6, 129.5, 128.5, 127.8, 127.2, 126.7, 120.8, 118.5, 99.4, 44.4, 25.9, 24.1, 22.6, 21.3, 18.7. LRMS (EI+) (m/z) (relative intensity): 396.2 (M+, 100), 397.2 (66), 381.2 (65). HRMS (TOF-ES)+ calcd for C27H29N2O (M + H): 397.2280, found 397.2280.
N-Benzyl-2-(5-methoxy-2-(methoxymethyl)-1H-indol-1-yl)benzamide (8o).
280 mg, 70% yield; brown oil, 1H NMR (400 MHz CDCl3) δH (ppm) 7.94–7.98 (m, 1H), 7.60–7.55 (m, 2H), 7.46 (dd, J = 7.6, 1.6 Hz, 1H), 7.20–7.17 (m, 1H), 7.12–7.03 (m, 4H), 6.80 (dd, J = 8.6, 2.2 Hz, 1H), 6.62 (d, J = 6.9 Hz, 2H), 6.49 (s, 1H), 6.38 (d, J = 1.7, 1H), 4.40 (d, J = 11.6, 1H), 4.20 (dd, J = 14.9, 6.1 Hz, 1H), 2.25–4.04 (dd, J = 14.8, 5.2 Hz, 1H), 3.68 (s, 3H), 2.91 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 166.7, 157.6, 140.2, 137.76, 136.7, 134.3, 134.2, 131.3, 130.9, 129.5, 129.3, 128.3, 127.5, 127.0, 121.7, 121.2, 111.6, 105.3, 93.5, 66.5, 57.9, 55.8, 43.9. LRMS (EI+) (m/z) (relative intensity): 400.2 (M+, 100), 191.1 (80), 250 (60). HRMS (TOF-ES)+ calcd for C25H24N2O3Na (M + Na): 423.1685, found 423.1689.
N-Benzyl-2-(6-methoxy-2-phenyl-1H-indol-1-yl)benzamide (8p).
272 mg, 63% yield; colorless solid, mp 130–132 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.06–8.03 (m, 1H), 7.53–7.48 (m, 3H), 7.26–7.24 (m, 1H), 7.18–7.14 (m, 4H), 7.12–7.09 (m, 3H), 6.84 (dd, J = 8.2,2.2 Hz, 1H), 6.74 (d, J = 6.8 Hz, 1H), 6.68 (s, 1H), 6.45 (d, J = 2.00, 1H), 4.42–4.41 (m, 2H), 3.68 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 166.3, 157.4, 140.1, 139.9, 137.3, 136.0, 133.9, 132.0, 131.9, 131.5, 130.4, 128.9, 128.63, 128.56, 128.34, 127.86, 127.6, 127.4, 122.7, 121.8, 111.6, 105.1, 104.5, 94.1, 55.8, 44.4. LRMS (EI+) (m/z) (relative intensity): 432.2 (M+, 100), 298.1 (32), 212.1 (22). HRMS (TOF-ES)+ calcd for C29H25N2O2 (M + H): 433.1913, found 433.1912.
N-Benzyl-2-(6-methyl-2-phenyl-1H-indol-1-yl)benzamide (8q).
278 mg, 67% yield; colorless solid, mp 175–177 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.05 (dd, J = 5.66, 2.0 Hz, 1H), 7.53–7.50 (m, 3H), 7.27 (dd, J = 5.76, 1.76 Hz, 1H), 7.19–7.09 (m, 7H), 7.01 (dd, J = 7.96, 0.84 Hz, 1H), 6.72–6.70 (m, 3H), 5.65 (bs, 1H), 4.24–4.10 (m, 2H), 2.35 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.3, 140.5, 139.7, 137.3, 136.1, 134.0, 133.4, 132.0, 131.9, 131.47, 130.57, 128.9, 128.6, 128.5, 128.5, 127.8, 127.7, 126.4, 123.4, 120.8, 110.6, 104.4, 44.4, 22.0. LRMS (EI+) (m/z) (relative intensity): 211.1 (100), 416.0 (M+, 85), 282.1 (65). HRMS (TOF-ES)+ calcd for C29H25N2O (M + H): 417.1967, found 417.1966.
N-Benzyl-2-(6-nitro-2-phenyl-1H-indol-1-yl)benzamide (8r).
339 mg, 76% yield; yellow solid, mp 182–184 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.55 (d, J = 2.2 Hz, 1H), 7.97 (dd, J = 9.6, 2.2 Hz, 1H), 7.81 (dd, J = 7.6, 1.8 Hz, 1H), 7.59–7.54 (m, 2H), 7.35 (dd, J = 7.5, 1.5 Hz, 1H), 7.27–7.14 (m, 9H), 7.02 (d, J = 9.2 Hz, 1H), 5.38 (bs, 1H), 6.88 (s, 1H), 6.85 (d, J = 6.8 Hz, 2H), 4.24–4.08 (m, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.4, 144.4, 142.9, 141.8, 137.1, 135.01, 134.98, 132.0, 130.9, 130.3, 129.6, 128.9, 128.7, 128.7, 128.1, 127.8, 127.6, 118.4, 118.0, 110.5, 105.4, 44.4. LRMS (EI+) (m/z) (relative intensity): 326.1 (100), 268.1 (98), 447.2 (M+, 62). HRMS (TOF-ES)+ calcd for C28H22N3O3 (M + H): 448.1661, found 448.1661.
N-Benzyl-2-(5-chloro-2-phenyl-1H-indol-1-yl)benzamide (8s).
186 mg, 55% yield; colorless solid, mp 161–163 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.93 (dd, J = 7.4, 2.5 Hz, 1H), 7.56 (d, J = 1.9 Hz, 1H), 7.52–7.49 (m, 1H), 7.27–7.25 (m, 1H), 7.22–7.13 (m, 8H), 7.05 (dd, J = 8.7, 2.1 Hz, 1H), 6.90 (d, J = 8.7 Hz, 1H), 6.75 (d, J = 0.5 Hz, 1H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.3, 142.4, 137.6, 137.1, 135.49, 134.4, 131.9, 131.3, 131.04, 130.36, 129.45, 129.20, 128.70, 128.64, 128.26, 127.89, 127.60, 127.04, 123.40, 120.45, 111.70, 103.71, 44.41. LRMS (EI+) (m/z) (relative intensity): 436.1 (M+, 100), 302.1 (68), 438.1 (38). HRMS (EI+) calcd for C28H21ClN2O (M+): 436.1342, found 436.1342.
2-(5-Chloro-2-phenyl-1H-indol-1-yl)-N-phenylbenzamide (8t).
257 mg, 61% yield; colorless solid, mp 197–199 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.96 (d, J = 7.8 Hz, 1H), 7.68 (d, J = 1.7 Hz, 1H), 7.65–7.61 (m, 1H), 7.55–7.52 (m, 1H), 7.47 (d, J = 7.7 Hz, 1H), 7.24–7.15 (m, 5H), 7.12–7.09 (m, 3H), 7.01 (t, J = 7.4 Hz, 1H), 6.93 (d, J = 8.0 Hz, 2H), 6.76 (s, 1H), 6.69 (bs, 1H). 13C NMR (100 MHz CDCl3) δC (ppm): 163.0, 143.0, 137.5, 137.2, 135.3, 134.4, 132.3, 131.5, 131.0, 130.1, 129.7, 129.3, 128.9, 128.8, 128.6, 128.4, 127.4, 124.5, 123.7, 120.5, 119.9, 111.7, 103.9. LRMS (EI+) (m/z) (relative intensity): 422.1 (M+, 100), 295.1 (46), 424.1 (38). HRMS (EI+) calcd for C27H19ClN2O (M+): 422.1186, found 422.1186.
2-(5-Chloro-2-heptyl-1H-indol-1-yl)-N-(3methoxypropyl)benzamide (8u).
295 mg, 67% yield; colorless solid, mp 66–68 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.14 (dd, J = 5.40, 2.6 Hz, 1H), 7.65–7.61 (m, 1H), 7.61–7.58 (m, 3H), 7.55 (d, J = 1.8 Hz, 1H), 7.29–7.26 (m, 1H), 6.84 (d, J = 8.6 Hz, 1H), 6.40 (s, 1H), 5.27 (bs, 1H), 3.1–3.02 (m, 5H), 2.97–2.85 (m, 2H), 2.55–2.38 (m, 2H), 1.62–1.51 (m, 2H), 1.31–1.13 (m, 10H), 0.84 (t, J = 6.9 Hz, 1H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.7, 144.1, 136.6, 135.0, 134.3131.8, 131.4, 130.0, 129.6, 129.5, 126.5, 122.1, 119.7, 110.8, 100.8, 58.7, 37.6, 31.8, 29.3, 29.1, 29.0, 28.3, 26.9, 22.7, 14.2. LRMS (EI+) (m/z) (relative intensity): 440.2 (M+, 100), 356.1 (64), 442.2 (34). HRMS (EI+) calcd for C26H33ClN2O2 (M+): 440.2231, found 440.2231.
N-Benzyl-2-(2-(2-bromo-4-chlorophenyl)-5-chloro-1H-indol-1-yl)benzamide (8v).
214 mg, 39% yield; colorless solid, mp 120–123 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 7.81 (d, J = 9.08 Hz, 1H), 7.62 (d, J = 1.4 Hz, 1H), 7.54 (d, J = 1.6 Hz, 1H), 7.46–7.43 (m, 2H), 7.30–7.06 (m, 6H), 6.90 (d, J = 6.7 Hz, 1H), 6.80 (d, J = 6.6 Hz, 2H), 6.79 (s, 2H), 5.61 (bs, 1H), 4.31–4.15 (m, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 165.6, 139.5, 137.0, 136.5, 135.5, 134.6, 134.5, 133.6, 132.7, 132.7, 131.7, 131.2, 130.5, 130.4, 129.3, 128.9, 128.8, 128.0, 127.8, 127.0, 125.0, 123.7, 120.9, 111.5, 105.9, 44.6. LRMS (EI+) (m/z) (relative intensity): 532.0 (100), 548.0 (M+, 60), 534.0 (55). HRMS (EI+) calcd for C28H19BrCl2N2O (M+): 548.0058, found 548.0057.
2-(2-(2-Bromo-4-chlorophenyl)-5-chloro-1H-indol-1-yl)-N-(3-methoxypropyl)benzamide (8w).
340 mg, 64% yield; colorless solid, mp 163–165 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 7.40 (dd, J = 6.2, 2.9 Hz, 1H), 7.67 (s, 1H), 7.57 (d, J = 1.6 Hz, 1H), 7.45–7.40 (m, 2H), 7.28–7.22 (m, 2H), 7.14 (dd, J = 5.5, 1.9 Hz, 2H), 7.00 (d, J = 8.6 Hz, 1H), 6.75 (s, 1H), 5.90 (bs, 1H), 3.23–3.12 (m, 6H), 1.41 (t, J = 6.06 Hz, 2H). 13C NMR (100 MHz, CDCl3) δC (ppm): 165.9, 139.5, 136.6, 135.4, 134.9, 134.6, 133.7, 132.7, 131.4, 130.2, 130.5, 130.1, 129.0, 127.5, 126.8, 125.0, 123.4, 120.7, 111.7, 105.9, 71.4, 58.9, 38.5, 28.8. LRMS (EI+) (m/z) (relative intensity): 532.0 (100), 530 (M+, 62), 534.0 (50). HRMS (EI+) calcd for C25H21BrCl2N2O2 (M+): 530.0163, found 530.0162.
3,12-Dichloro-N-(3-methoxypropyl)indolo[1,2-f]phenanthridine-8-carboxamide (10).
226 mg, 85% yield; colorless solid, mp 271–273 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.31 (d, J = 7.8 Hz, 1H), 8.18 (s, 1H), 8.0–8.04 (m, 2H), 7.89 (d, J = 9.1 Hz, 1H), 7.71 (s, 1H), 7.48 (d, J = 7.7, 2H), 7.24 (d, J = 8.1 Hz, 1H), 7.16 (s, 1H), 6.08 (bs, 1H), 3.14 (d, J = 4.9 Hz, 2H), 2.97 (s, 5H), 1.21 (s, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 167.5, 136.1, 134.8, 134.6, 131.8, 131.2, 130.6, 129.3, 128.4, 128.2, 127.4, 125.6, 125.3, 124.5, 124.4, 123.8, 123.0, 122.4, 120.0, 114.6, 97.8, 72.1, 58.8, 39.4, 27.9. LRMS (EI+) (m/z) (relative intensity): 450.1 (M+, 100), 327.0 (78), 452.1 (66), 362.0 (60). HRMS (EI+) calcd for C25H20BrCl2N2O2 (M+): 450.0902, found 450.0900.
3-(2-Aminobenzylidene)-2-methylisoindolin-1-one (4a).
180 mg, 72% yield; yellow solid, mp 174–176 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 7.83 (d, J = 7.5 Hz, 1H), 7.74 (d, J = 7.6 Hz, 1H), 7.57 (t, J = 7.5 Hz, 1H), 7.47 (t, J = 7.44, 1H), 7.16–7.09 (m, 2H), 6.79–6.72 (m, 2H), 6.51 (s, 1H), 3.87 (bs, 2H), 3.04 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.7, 145.0, 137.7, 137.6, 132.0, 130.9, 129.2, 128.7, 123.7, 119.9, 119.5, 118.1, 115.1, 102.5, 29.3. LRMS (+ESI) (m/z) (relative intensity): 251.46 (M + H, 100), 220.58. HRMS (EI+) calcd for C16H14N2O (M+): 250.1106, found 250.1107.
3-(2-Amino-4-bromobenzylidene)-2-methylisoindolin-1-one (4b).
243 mg, 74% yield; yellow solid, mp 193–195 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.85 (d, J = 7.5 Hz, 1H), 7.75 (d, J = 7.7 Hz, 1H), 7.60 (t, J = 7.4 Hz, 1H), 7.50 (t, J = 7.5 Hz, 1H), 6.97 (d, J = 8.5 Hz, 2H), 6.70 (d, J = 6.2 Hz, 1H), 6.39 (s, 1H), 3.89 (bs, 2H), 3.05 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.7, 146.2, 137.5, 137.6, 132.2, 132.1, 129.5, 128.8, 123.4, 122.8, 121.1, 119.6, 119.5, 118.9, 117.7, 100.8, 29.3. LRMS (EI+) (m/z) (relative intensity): 297.0 (100), 328.0 (M+, 92), 330.0 (88). HRMS (EI+) calcd for C16H13BrN2O (M+): 328.0211, found 328.0211.
3-(2-Amino-5-chlorobenzylidene)-2-methylisoindolin-1-one (4c).
210 mg, 74% yield; yellow solid, mp 163–165 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.85 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.51 (t, J = 2.4 Hz, 1H), 7.11 (t, J = 2.6 Hz, 2H), 6.67 (d, J = 9.2 Hz, 1H), 6.42 (s, 1H), 3.86 (s, 2H), 3.07 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.7, 143.6, 137.4, 138.5, 137.4, 132.2, 130.3, 129.6, 129.0, 128.8, 123.4, 122.8, 121.4, 119.6, 116.24, 100.7, 29.3. LRMS (+ESI) (m/z) (relative intensity): 285.52 (M + H, 100), 287.36 (38), 254.47 (28). HRMS (EI+) calcd for C16H13ClN2O (M+): 284.0716, found 284.0716.
3-(2-Amino-5-chlorobenzylidene)-2-ethylisoindolin-1-one (4d).
232 mg, 78% yield; yellow solid, mp 172–174 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.85 (d, J = 7.52 Hz, 1H), 7.75 (d, J = 7.72, 1H), 7.60 (t, J = 7.54, 1H), 7.51 (t, J = 7.48, 1H), 7.06 (t, J = 7.76 Hz, 1H), 6.76 (t, J = 4.62 Hz, 2H), 6.39 (s, 1H), 3.92 (s, 2H), 0.86 (t, J = 7.48, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.5, 145.9, 137.9, 136.8, 134.8, 132.1, 131.8, 129.4, 128.9, 123.4, 119.6, 118.2, 118.1, 114.7, 100.8, 36.0, 13.7. LRMS (EI+) (m/z) (relative intensity): 253.0 (100), 298.1 (M+, 75), 300.1(28). HRMS (EI+) calcd for C17H15ClN2O (M+): 298.0873, found 298.0873.
(2-Allyl-3-(2-amino-5-chlorobenzylidene)isoindolin-1-one (4e).
232 mg, 75% yield; yellow solid, mp 132–134 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.87 (d, J = 7.5 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.62 (t, J = 7.5 Hz, 1H), 7.52 (t, J = 7.4 Hz, 1H), 7.11 (t, J = 5.3 Hz, 2H), 6.65 (d, J = 8.2 Hz, 1H), 6.39 (s, 1H), 5.49–5.43 (m, 1H), 4.93 (d, J = 10.4, 1H), 4.6 (d, J = 17.1 Hz, 1H), 4.30 (d, J = 4.9 Hz, 1H), 3.79 (bs, 1H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.9, 143.6, 137.8, 137.1, 132.3, 130.3, 129.5, 129.0, 129.2, 128.5, 123.5, 122.7, 121.2, 119.6, 116.3, 100.7, 69.5, 58.6, 40.2. LRMS (+ESI) (m/z) (relative intensity): 311.52 (M + H, 100), 313.31 (37). HRMS (EI+) calcd for C18H15ClN2O (M+): 310.0873, found 310.0875.
3-(2-Amino-5-chlorobenzylidene)-2-(2-methoxyethyl)isoindolin-1-one (4f).
229 mg, 71% yield; yellow solid, mp 132–134 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.86 (d, J = 7.5 Hz, 1H), 7.75 (d, J = 7.72 Hz, 1H), 7.61 (t, J = 7.3 Hz, 1H), 7.51 (t, J = 7.34 Hz, 1H), 7.51 (t, J = 7.4 Hz, 1H), 7.13–7.09 (m, 2H), 6.67 (d, J = 8.4 Hz, 1H), 6.40 (s, 1H), 3.90 (t, J = 6.1 Hz, 2H), 3.84 (s, 2H), 3.24 (t, J = 6.0 Hz, 2H), 3.1 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.9, 143.6, 137.8, 137.1, 132.3, 130.3, 129.5, 129.2, 128.5, 123.5, 122.7, 121.2, 119.6, 116.3, 100.7, 69.5, 58.6, 40.2. LRMS (+ESI) (m/z) (relative intensity): 351.64 (M + Na, 100), 353.51 (33). HRMS (EI+) calcd for C18H17ClN2O2 (M+): 328.0979, found 328.0981.
3-(2-Aminobenzylidene)-2-phenylisoindolin-1-one (4g).
193 mg, 62% yield; yellow solid, mp 175–177 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.95 (d, J = 7.5 Hz, 1H), 7.88 (d, J = 7.7 Hz, 1H), 7.68 (t, J = 7.5 Hz, 1H), 7.56 (t, J = 7.4 Hz, 1H), 7.11 (t, J = 6.8 Hz, 5H), 6.8 (t, J = 7.5 Hz, 1H), 6.62 (s, 1H), 6.49 (d, J = 6.5 Hz, 1H), 6.42 (d, J = 7.6 Hz, 1H), 6.22 (t, J = 6.6 Hz, 1H), 3.68 (bs, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.0, 143.7, 138.2, 135.8, 135.4, 132.6, 130.6, 129.4, 129.0, 129.0, 128.4, 128.1, 128.04, 127.42, 127.0, 124.0, 119.7, 119.3, 117.8, 114.7, 103.5. LRMS (+ESI) (m/z) (relative intensity): 313.42 (M + H, 100), 181.06 (13). HRMS (EI+) calcd for C21H16N2O (M+): 312.1263, found 312.1262.
3-(2-Amino-4-bromobenzylidene)-6-bromo-2-phenylisoindolin-1-one (4h).
300 mg, 64% yield; yellow solid, mp 212–214 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.05 (d, J = 1.2 Hz, 1H), 7.77 (dd, J = 8.2, 1.52 Hz, 1H), 7.70 (d, J = 8.3 Hz, 1H), 7.11–7.09 (m, 3H), 7.02–7.00 (m, 2H), 6.56 (d, J = 1.4 Hz, 1H), 6.45 (s, 1H), 6.33–6.27 (m, 2H), 3.75 (bs, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 166.5, 144.9, 136.6, 135.7, 135.6, 134.9, 131.7, 129.8, 129.0, 128.8, 128.3, 127.5, 127.3, 127.1, 123.6, 122.2, 121.3, 120.6, 117.8, 117.3, 102.9. LRMS (EI+) (m/z) (relative intensity): 376.9 (100), 470.0 (85), 299.0 (72), 468.0 (M+, 44). HRMS (EI+) calcd for C21H14Br2N2O (M+): 467.9473, found 467.9475.
3-(2-Amino-5-chlorobenzylidene)-6-bromo-2-phenylisoindolin-1-one (4i).
289 mg, 68% yield; yellow solid, mp 212–214 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.08 (d, J = 1.4 Hz, 1H), 7.79 (dd, J = 8.2, 1.6 Hz, 1H), 7.72 (d, J = 8.2 Hz, 1H), 7.16–7.03 (m, 5H), 6.74 (dd, J = 8.5, 2.3 Hz, 1H), 6.46 (s, 1H), 6.41 (d, J = 2.1 Hz, 1H), 6.35 (d, J = 8.6 Hz, 1H), 3.69 (s, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 166.4, 142.4, 136.7, 136.2, 135.7, 130.4, 129.9.3, 128.4, 127.8, 127.3, 127.2, 123.8, 122.5, 121.3, 120.23, 115.84, 102.54. LRMS (EI+) (m/z) (relative intensity): 93.0 (100), 426.0 (82), 332.9 (80), 424.0(M+, 64). HRMS (EI+) calcd for C21H14BrClN2O (M+): 423.9978, found 423.9980.
3-(2-Amino-4-methoxybenzylidene)-2-phenylisoindolin-1-one (4j).
280 mg, 82% yield; yellow solid, mp 196–198 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.49 (d, J = 7.6 Hz, 1H), 7.86 (d, J = 7.8 Hz, 1H), 7.68–7.64 (m, 1H), 7.53 (t, J = 7.5 Hz, 1H), 7.12–7.05 (m, 5H), 6.57 (s, 1H), 6.37 (s, 2H), 5.99 (s, 3H), 5.85 (q, J = 7.5 Hz, 1H), 3.72 (s, 2H), 3.63 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.0, 160.2, 145.0, 138.4, 135.6, 135.0, 132.5, 131.7, 129.2, 128.1, 128.0, 127.4, 126.9, 124.0, 119.6, 112.4, 104.0, 103.3, 100.2, 55.3. LRMS (+ESI) (m/z) (relative intensity): 343.52 (M + H, 100), 181.20 (12). HRMS (EI+) calcd for C22H18N2O2 (M+): 342.1368, found 342.1369.
3-(2-Amino-5-chlorobenzylidene)-2-phenylisoindolin-1-one (4k).
245 mg, 71% yield; yellow solid, mp 163–165 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.85 (d, J = 9.0 Hz, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.51 (t, J = 2.4 Hz, 1H), 7.11 (t, J = 2.6 Hz, 2H), 6.67 (d, J = 9.2 Hz, 1H), 6.42 (s, 1H), 3.86 (s, 2H), 3.07 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm): 167.9, 142.4, 137.9, 136.9, 135.3, 132.7, 130.4, 129.8, 128.3, 128.2, 128.2, 127.6, 127.4, 124.1, 122.4, 120.6, 119.7, 115.7, 101.6. LRMS (EI+) (m/z) (relative intensity): 253 (100), 219.1 (72), 93.1 (64), 346.1 (M+, 65). HRMS (EI+) calcd for C21H15ClN2O (M+): 346.0873, found 346.0871.
3-(2-Aminobenzylidene)-2-benzylisoindolin-1-one (4l).
234 mg, 72% yield; yellow solid, mp 169–171 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.94 (d, J = 7.5, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.62 (t, J = 7.4 Hz, 1H), 7.54 (t, J = 7.4 Hz, 1H), 7.14–7.02 (m, 4H), 6.97 (d, J = 7.4 Hz, 1H), 6.73 (t, J = 7.4 Hz, 1H), 6.57–6.51 (m, 3H), 6.41 (s, 1H), 4.96 (s, 2H), 3.19 (s, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 169.0, 145.5, 138.2, 137.3, 135.8, 132.3, 131.2, 129.3, 129.0, 128.4, 128.1, 126.9, 126.6, 123.8, 120.4, 119.8, 118.2, 115.4, 103.8, 44.72. LRMS (+ESI) (m/z) (relative intensity): 327.54 (M + H, 100), 220.52 (24). HRMS (EI+) calcd for C22H18N2O (M+): 326.1419, found 326.1419.
3-(2-Amino-4-bromobenzylidene)-2-benzylisoindolin-1-one (4m).
291 mg, 72% yield; yellow solid, mp 169–171 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.94 (d, J = 7.5 Hz, 1H), 7.74 (d, J = 7.6 Hz, 1H), 7.63 (t, J = 7.5 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.11–7.06 (m, 3H), 6.83–6.70 (m, 2H), 6.69 (s, 1H), 6.56 (d, J = 6.7 Hz, 2H), 6.27 (s, 1H), 4.93 (s, 2H), 3.27 (s, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 168.9, 146.8, 138.0, 137.0, 136.4, 132.5, 132.3, 129.5, 128.3, 128.2, 127.1, 126.3, 123.9, 122.7, 120.9, 119.8, 119.2, 117.8, 102.3, 44.7. LRMS (EI+) (m/z) (relative intensity): 254.1 (100), 406.1 (64), 404.1 (M+, 63). HRMS (EI+) calcd for C22H17BrN2O (M+): 404.0524, found 404.0526.
3-(2-Amino-5-chlorobenzylidene)-6-bromo-2-phenylisoindolin-1-one (4n).
294 mg, 67% yield; yellow solid, mp 209–211 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.06 (s, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.09 (t, J = 9.3 Hz, 4H), 6.83 (s, 1H), 6.49 (t, J = 9.1 Hz, 3H), 6.28 (s, 1H), 4.93 (s, 2H), 3.24 (s, 2H). 13C NMR (100 MHz CDCl3) δC (ppm): 167.4, 144.0, 138.6, 136.5, 135.8, 135.5, 130.4, 130.0, 129.2, 128.3, 129.2, 128.3, 127.2, 127.0, 126.3, 123.7, 122.9, 121.4, 121.4, 116.4, 103.0, 44.9. LRMS (+ESI) (m/z) (relative intensity): 439.41 (M + H, 76), 441.34 (100). HRMS (EI+) calcd for C22H16BrClN2O (M+): 438.0135, found 438.0135.
N-Methyl-2-((2-((2-methyl-3-oxoisoindolin-1-ylidene)methyl)phenyl)amino)benzamide (5a).
Yield 74%, 210 mg; yellow solid, mp 189–191 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 9.33 (s, 1H), 7.82 (d, J = 7.4 Hz, 1H), 7.76 (d, J = 7.7 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.46–7.42 (m, Hz, 2H), 7.37–7.26 (m, 6H), 7.04 (t, J = 7.4 Hz, 1H), 6.78 (t, J = 7.2 Hz, 1H), 6.66 (s, H), 6.10 (bs, 1H), 3.06 (s, 3H), 2.88 (d, J = 4.7 Hz, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.0, 169.1, 144.9, 141.5, 138.2, 137.9, 132.0, 131.6, 129.3, 129.0, 128.7, 128.6, 127.6, 127.5, 127.0, 123.2, 123.1, 121.8, 119.8, 119.68, 119.53, 118.83, 116.50, 107.00, 102.9, 30.2, 26.8. LRMS (+ESI) (m/z) (relative intensity): 406.63 (M + Na, 100), 381.70 (14). HRMS (EI+) calcd for C24H21N3O2 (M+): 383.1634, found 383.1634.
1-Acetyl-3-bromo-2′-methylspiro[indoline-2,1′-isoindolin]-3′-one (6a).
152 mg, 82% yield; colorless solid, mp 173–175 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 9.33 (s, 1H), 8.25 (d, J = 6.6 Hz, 1H), 7.95 (d, J = 6.9 Hz, 1H), 7.71–7.62 (m, 2H), 7.54 (d, J = 7.4 Hz, 1H), 7.41 (t, J = 8.3 Hz, 2H), 7.23 (t, J = 7.5 Hz, 1H), 5.86 (s, 1H), 2.80 (s, 3H), 1.57 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.0, 166.8, 144.1, 142.2, 133.4, 132.6, 133.9, 130.8, 127.1, 125.2, 124.5, 121.3, 117.0, 86.2, 57.3, 25.9, 24.0. LRMS (+ESI) (m/z) (relative intensity): 393.40 (M + Na, 95), 395.30 (100). HRMS (EI+) calcd for C18H15BrN2O2 (M+): 370.0317, found 370.0316.
1-Acetyl-3,6-dibromo-2′-methylspiro[indoline-2,1′-isoindolin]-3′-one (6b).
184 mg, 82% yield; colorless solid, mp 195–197 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.51 (s, 1H), 7.95 (dd, J = 6.68, 1.06 Hz, 1H), 7.72–7.63 (m, 2H), 7.53 (d, J = 8.00 Hz, 1H), 7.37 (dd, J = 8.04, 1.72 Hz, 1H), 7.27 (dd, J = 8.04, 1.72 Hz, 1H), 5.76 (s, 1H), 2.81 (s, 3H), 1.53 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.2, 166.6, 143.7, 143.1, 133.5, 132.5, 131.0, 128.2, 126.2, 126.1, 124.6, 124.5, 121.3, 120.3, 86.5, 56.4, 25.9, 23.8. LRMS (EI+) (m/z) (relative intensity): 447.94 (M+, 15), 371 (100), 227 (97). HRMS (TOF-ES)+ calcd for C18H15N2O2Br2 (M + H): 448.9500, found 448.9504.
1-Acetyl-3-bromo-5-chloro-2′-methylspiro[indoline-2,1′-isoindolin]-3′-one (6c).
172 mg, 85% yield; colorless solid, mp 178–182 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.23 (d, J = 8.6 Hz, 1H), 7.94 (d, J = 7.1 Hz, 1H), 7.71–7.64 (m, 2H), 7.54 (d, J = 7.4 Hz, 1H), 7.38–7.33 (m, 2H), 5.8 (s, 1H), 2.79 (s, 3H), 1.52 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.1, 166.7, 143.7, 140.85, 133.5, 132.5, 131.0, 130.8, 130.2, 128.9, 125.2, 124.6, 121.3, 118.3, 86.4, 56.0, 25.9, 23.8. LRMS (EI+) (m/z) (relative intensity): 429.14 (100), 427 (M + Na, 86), 296.1 (38). HRMS (TOF-ES)+ calcd for C18H15N2O2ClBr (M + H): 405.0005, found 405.0007.
1-Acetyl-3-bromo-5-chloro-2′-ethylspiro[indoline-2,1′-isoindolin]-3′-one (6d).
175 mg, 84% yield; colorless solid, mp 193–195 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.38 (s, 1H), 7.94 (d, J = 1.1 Hz, 1H), 7.72–7.62 (m, 2H), 7.54 (d, J = 7.36 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.21 (dd, J = 7.36, 1.2 Hz, 1H), 5.83 (s, 1H), 3.95–3.86 (m, 1H), 2.75–2.66 (m, 1H), 1.56 (s, 3H), 1.07 (t, J = 7.20 Hz, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.2, 166.7, 144.0, 143.0, 136.6, 133.5, 132.5, 131.0, 125.9, 125.7, 125.3, 124.6, 121.2, 117.8, 87.2, 56.9, 36.5, 24.1, 13.9. LRMS (EI+) (m/z) (relative intensity): 418.01 (M+, 46), 420.0 (68), 339.1 (100). HRMS (TOF-ES)+ calcd for C19H17N2O2ClBr (M + H): 419.0162, found 419.0160.
1-Acetyl-3-bromo-5-chloro-2′-(methoxyethyl)spiro[indoline-2,1′-isoindolin]-3′-one (6e).
199 mg, 89% yield; colorless solid, mp 180–183 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.30 (d, J = 4.7 Hz, 1H), 7.93 (d, J = 7.4 Hz, 1H), 7.72–7.62 (m, 2H), 7.55 (d, J = 7.4 Hz, 1H), 7.35–7.33 (m, 2H), 5.85 (s, 1H), 4.04–3.98 (m, 1H), 3.62–3.57 (m, 1H), 3.30–3.26 (m, 1H), 3.15 (s, 1H), 2.81–2.74 (m, 1H), 1.51 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.5, 167.0, 144.1, 141.5, 136.6, 133.4, 132.0, 130.7, 130.6, 129.5, 128.5, 124.6, 124.3, 121.2, 118.5, 86.6, 69.6, 58.4, 57.1, 42.2. LRMS (+ESI) (m/z) (relative intensity): 471.50 (M + Na, 74), 473.34 (100). HRMS (EI+) calcd for C20H18BrClN2O3 (M+): 448.0189, found 448.0188.
1-Acetyl-3,5-dichloro-2′-(methoxyethyl)spiro[indoline-2,1′-isoindolin]-3′-one (6f).
180 mg, 89% yield; colorless solid, mp 192–194 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 7.93 (d, J = 6.1 Hz, 1H), 7.69 (d, J = 5.8 Hz, 2H), 7.64 (d, J = 6.2 Hz, 1H), 7.36 (s, 2H), 5.73 (s, 1H), 3.99 (d, J = 14.2 Hz, 1H), 3.59 (s, 1H), 3.28 (s, 1H), 3.15 (s, 3H), 2.8 (d, J = 9.3 Hz, 1H), 1.50 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.8, 167.4, 144.5, 141.5, 133.6, 132.0, 130.9, 129.7, 128.5, 124.5, 124.0, 121.4, 118.8, 87.2, 69.9, 66.3, 58.6, 42.5, 24.1. LRMS (+ESI) (m/z) (relative intensity): 427.58 (M + Na, 100), 429.32 (63). HRMS (EI+) calcd for C20H18Cl2N2O3 (M+): 404.0694, found 404.0695.
1-Acetyl-2′-benzyl-3-bromospiro[indoline-2,1′-isoindolin]-3′-one (6h).
158 mg, 77% yield; colorless solid, mp 174–177 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.07 (s, 1H), 7.91 (dd, J = 6.68, 1.20 Hz, 1H), 7.56 (d, J = 7.16 Hz, 2H), 7.40 (t, J = 8.38 Hz, 3H), 7.21 (t, J = 8.2 Hz, 1H), 7.20–6.88 (m, 3H), 6.87 (s, 1H), 5.82 (s, 1H), 5.25 (d, J = 15.0 Hz, 3H), 3.49 (d, J = 15.2 Hz, 1H), 0.97 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 169.7, 167.2, 144.4, 142.6, 136.6, 133.6, 132.1, 130.8, 128.7, 128.5, 128.3, 128.1, 127.0, 125.1, 124.8, 124.7, 121.2, 118.1, 86.6, 58.4, 45.0, 23.7. LRMS (EI+) (m/z) (relative intensity): 446.1 (M+, 45), 367.0 (100), 220 (82). HRMS (TOF-ES)+ calcd for C24H20N2O2Br (M + H): 447.0708, found 447.0709.
1-Acetyl-2′-benzyl-3,6-dibromospiro[indoline-2,1′-isoindolin]-3′-one (6i).
205 mg, 78% yield; colorless solid, mp 163–165 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.28 (s, 1H), 7.98 (d, J = 5.0 Hz, 1H), 7.65 (s, 2H), 7.44–7.38 (m, 3H), 7.21 (s, 3H), 6.91 (s, 2H), 5.79 (s, 1H), 5.36 (d, J = 14.5 Hz, 3H), 1.07 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 167.1, 143.9, 136.2, 133.6, 132.0, 130.9, 128.8, 128.5, 128.2, 128.0, 126.1, 125.8, 124.7, 124.4, 121.1, 86.9, 57.2, 44.9, 23.8. LRMS (+ESI) (m/z) (relative intensity): 547.11 (M + Na, 44), 549.14 (100). HRMS (TOF-ES)+ calcd for C23H19N2O2Br2 (M + H): 524.9813, found 524.9811.
1-Acetyl-2′-benzyl-3,5′-dibromo-5-chlorospiro[indoline-2,1′-isoindolin]-3′-one (6j).
215 mg, 77% yield; colorless solid, mp 210–212 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 8.10 (d, J = 0.6 Hz, 1H), 8.00 (s, 1H), 7.79 (s, 1H), 7.46 (s, 1H), 7.3 (bs, 1H), 7.2–7.16 (m, 3H) 6.92 (s, 2H), 5.78 (s, 1H), 5.34 (d, J = 14.7 Hz, 1H), 3.53 (d, J = 15.3 Hz, 1H), 1.07 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 169.3, 165.6, 142.7, 141.0, 136.7, 136.0, 135.8, 133.9, 131.1, 130.8, 130.3, 128.9, 128.2, 128.6, 128.1, 128.0, 127.5, 126.0, 124.9, 122.7, 119.2, 86.6, 56.7, 45.1, 23.8. LRMS (+ESI) (m/z) (relative intensity): 581.06 (M + Na, 29), 583.08 (100). HRMS (TOF-ES)+ calcd for C24H18N2O2ClBr2 (M + H): 558.9424, found 558.9426.
1-Acetyl-3-bromo-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one (6k).
134 mg, 62% yield; colorless solid, mp 181–185 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.23 (s, 1H), 8.00 (s, 1H), 7.99 (dd, J = 5.3, 2.2 Hz, 1H), 7.65–7.59 (m, 2H), 7.41–7.25 (m, 8H) 7.10 (t, J = 7.5 Hz, 1H), 5.78 (s, 1H), 1.89 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.3, 167.6, 144.1, 142.6, 134.6, 133.2, 131.0, 130.8, 130.0, 128.4, 127.4, 127.0, 125.8, 124.9, 124.4, 123.6, 117.2, 89.5, 52.7, 24.2. LRMS (EI+) (m/z) (relative intensity): 432.0 (M+, 34), 353.1 (100), 281.1 (52). HRMS (TOF-ES)+ calcd for C23H18N2O2Br (M + H): 433.0552, found 433.0556.
1-Acetyl-3,5′,6-tribromo-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one (6l).
195 mg, 66% yield; colorless solid, mp 190–193 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.40 (s, 1H), 8.13 (d, J = 1.3 Hz, 1H), 7.76–7.74 (m, 1H), 7.56 (t, J = 7.22 Hz, 2H), 7.35–7.29 (m, 1H) 7.26–7.20 (m, 2H), 7.17 (s, 2H), 7.10 (d, J = 8.0 Hz, 1H), 5.66 (s, 1H), 1.93 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm): 169.9, 165.9, 143.2, 142.4, 136.3, 134.0, 132.6, 128.9, 128.8, 128.5, 128.1, 127.7, 127.0, 126.9, 126.2, 125.5, 125.2, 124.8, 120.4, 89.9, 51.5, 24.3. LRMS (EI+) (m/z) (relative intensity): 587.86 (M+, 22), 510.9 (100), 508 (94), 388 (84). HRMS (TOF-ES)+ calcd for C23H16N2O2Br3 (M + H): 588.8762, found 588.8759.
1-Acetyl-3,5′-dibromo-5-chloro-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one (6m).
169 mg, 62% yield; colorless solid, mp 193–195 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.15 (s, 1H), 7.86 (bs, 2H), 7.49 (bs, 1H), 7.25–7.10 (m, 7H), 5.83 (s, 1H), 1.73(bs, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 169.3, 165.2, 142.2, 141.0, 136.9, 134.0, 130.1, 129.8, 128.9, 128.4, 128.1, 125.8, 125.3, 125.0, 124.4, 122.9, 118.2, 114.3, 88.3, 57.1, 24.1. LRMS (+ESI) (m/z) (relative intensity): 566.82 (M + Na, 100), 568.81 (100). HRMS (TOF-ES)+ calcd for C23H15N2O2ClBr2 (M + H): 544.9267, found 544.9271.
1-Acetyl-3-bromo-5-chloro-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one (6n).
81 mg, 35% yield; colorless solid, mp 165–167 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.23 (s, 1H), 8.01–7.99 (m, 1H), 7.67–7.62 (m, 2H), 7.44–7.40 (m, 2H), 7.35–7.27 (m, 5H) 7.23 (s, 1H), 5.71 (s, 1H), 1.86 (s, 3H). 13C NMR (100 MHz CDCl3) δC (ppm): 170.3, 167.5, 143.8, 141.2, 134.4, 133.5, 131.3, 130.8, 130.7, 130.2, 130.0, 129.2, 128.6, 127.0, 125.8, 124.5, 123.6, 118.6, 90.1, 51.4, 24.0. LRMS (+ESI) (m/z) (relative intensity): 489.08 (M + Na, 82), 491.03 (100). HRMS (TOF-ES)+ calcd for C23H17N2O2ClBr (M + H): 467.0162, found 467.0164.
1-Acetyl-3-bromo-5-chloro-2′-phenylspiro[indoline-2,1′-isoindolin]-3′-one (6n1).
68 mg, 29% yield; colorless solid, mp 168–190 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.03 (d, J = 7.3 Hz, 1H), 8.00–7.95 (m, 1H), 7.75–7.63 (m, 3H), 7.22–7.11 (m, 8H), 5.89 (s, 1H), 1.68 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm): 169.8, 166.8, 143.7, 141.2, 134.0, 132.2, 131.2, 130.2, 129.9, 128.9, 128.3, 125.2, 124.5, 121.34, 118.3, 88.6, 57.4, 24.7. LRMS (EI+) (m/z) (relative intensity): 466.0 (M + H, 24), 387.1 (72), 345.1 (100). HRMS (EI+) calcd for C23H17N2O2ClBr (M+): 466.0084, found 466.0083.
1-Acetyl-6-methoxy-3′-oxo-2′-phenylspiro[indoline-2,1′-isoindolin]-3yl acetate(6o).
196 mg, 88% yield; colorless solid, mp 143–145 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.02–8.00 (m, 1H), 7.67–7.65 (m, 2H), 7.53–7.51 (m, 1H), 7.32 (d, J = 4.4 Hz, 4H), 7.28–7.25 (m, 1H) 7.01 (d, J = 8.4 Hz, 1H), 6.60 (dd, J = 8.36, 2.44 Hz, 1H), 6.31 (s, 1H), 3.84 (s, 3H), 1.77 (s, 3H), 1.70 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm): 169.7, 167.0, 162.1, 146.3, 141.3, 134.6, 132.6, 132.4, 130.7, 129.6, 128.3, 127.4, 125.8, 124.8, 124.7, 117.0, 111.2, 103.2, 89.2, 55.8, 24.3, 20.4. LRMS (+ESI) (m/z) (relative intensity): 464.99 (M + Na, 100), 383.11 (9). HRMS (TOF-ES)+ calcd for C26H23N2O5 (M + H): 443.1607, found 443.1611.
1-Acetyl-2′-allyl-3-bromo-5-chlorospiro[indoline-2,1′-isoindolin]-3′-one (6p).
155 mg, 72% yield; colorless solid, mp 171–173 °C; 1H NMR (400 MHz CDCl3) δH (ppm) 8.23 (s, 1H), 7.95 (d, J = 1.3 Hz, 1H), 7.72–7.62 (m, 2H), 7.54 (d, J = 7.3 Hz, 1H), 7.40 (s, 1H), 7.37–7.34 (m, 1H), 7.26 (s, 1H), 5.83 (s, 1H), 5.75–5.65 (m, 1H), 4.97 (d, J = 10.0 Hz, 1H), 4.7 (d, J = 17.0 Hz, 1H), 3.1 (q, J = 8.8 Hz, 1H), 1.50 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm): 169.9, 166.4, 143.9, 141.1, 133.6, 132.3, 131.6, 131.0, 130.6, 130.1, 128.9, 124.9, 124.7, 121.2, 119.1, 118.7, 86.8, 56.6, 43.8, 24.2. LRMS (+ESI) (m/z) (relative intensity): 453.33 (M + Na, 74), 455.23 (100). HRMS (EI+) calcd for C20H16BrClN2O2 (M+): 430.0084, found 430.0085.
Conclusions
In conclusion, we have developed an easy access for the synthesis of spiro fused isoindolinone-indolines utilizing a well-known, halogen sources mediated cyclopropylhalonium ion strategy. Also, we successfully utilized the ortho-effect of amide group in N-substituted 2-iodo benzamides for the ligand free synthesis of 1,2-substituted indolylcarboxamides which also provide a path for the synthesis of indolophenanthradinecarboxamide derivative.
Acknowledgements
Financial support by the Ministry of Science and Technology of the Republic of China (MOST 103-2113-M-003-008-MY3), National Taiwan Normal University (103-07-C) and Instrumentation Centre at National Taiwan Normal University is gratefully acknowledged. The authors are grateful to Ms Hsiu-Ni Huan, Ms Chiu-Hui He and Ting-Shen Kuo for providing HRMS, NMR spectral and Crystallographic data respectively.
Notes and references
- D. Cheng, Y. Ishihara, B. Tan and C. F. Barbasc III, ACS Catal., 2014, 4, 743–762 CrossRef CAS.
-
(a)
O. Panknin, S. Ring, S. Baurle, A. Wagenfeld, R. Nubbemeyer, K. Nowak-Reppel and G. Langer, US Pat., US20160052936, 2016;
(b)
J. Wendt, M. Cox, M. K. S. Sheehan, M. P. Curtis, T. Respondek, R. A. Ewin, G. M. Kyne and P. D. Johnson, World Patent, WO 2015100232 A3, 2015;
(c) N. A. Powell, J. T. Kohrt, K. J. Filipski, M. Kaufman, D. Sheehan, J. E. Edmunds, A. Delaney, Y. Wang, F. Bourbonais, D.-Y. Lee, F. Schwende, F. Sun, P. McConnell, C. Catana, H. Chen, J. Ohren and L. A. Perrin, Bioorg. Med. Chem. Lett., 2012, 22, 190–193 CrossRef CAS PubMed;
(d) A. H. Abdel-Rahman, E. M. Keshk, M. A. Hanna and S. M. El-Bady, Bioorg. Med. Chem., 2004, 12, 2483–2488 CrossRef CAS PubMed;
(e)
G. Iftime, P. M. Kazmaier, J. D. Mayo, P. F. Smith, I. Dorziotis, I. Houpis, A. Molina and R. Volante, World Patent, WO 1998018815 A1, 1998;
(f) P. E. Maligres, I. Houpis, K. Rossen, A. Molina, J. Sager, V. Upadhyay, K. M. Wells, R. A. Reamer, J. E. Lynch, D. Askin, R. P. Volante and P. J. Reider, Tetrahedron, 1997, 53, 10983–10992 CrossRef CAS.
-
L.-P. Molleyres, J. Cassayre, F. Cederbaum and P. Maienfisch, World Patent, WO 2005061500 A1, 2005.
-
(a) A. Mertens, H. Zilch, B. König, W. Schafer, T. Poll, W. Kampe, H. Seidel, U. Leser and H. Leinert, J. Med. Chem., 1993, 36, 2526 CrossRef CAS PubMed;
(b) J. Wrobel, A. Dietrich, S. A. Woolson, J. Millen, M. McCaleb, M. C. Harrison, T. C. Hohman, J. Sredy and D. Sullivan, J. Med. Chem., 1992, 35, 4613 CrossRef CAS PubMed;
(c) X. Bao, J. Shi, X. Nie, B. Zhou, X. Wang, L. Zhang, H. Liao and T. Peng, Bioorg. Med. Chem., 2014, 22, 4826 CrossRef CAS PubMed.
-
(a) Z.-Y. Tang and Q.-S. Hu, Adv. Synth. Catal., 2006, 348, 846–850 CrossRef CAS;
(b) C. B. Lavery, R. McDonald and M. Stradiotto, Chem. Commun., 2012, 7277 RSC;
(c) Y. Zheng, J. Cleaveland, D. Richardson and Y. Yuan, Org. Lett., 2015, 17, 4240 CrossRef CAS PubMed;
(d) R. K. Nandi, R. Guillot, C. Kouklovsky and G. Vincent, Org. Lett., 2016, 18, 1716 CrossRef CAS PubMed;
(e) S. Karamthulla, S. Pal, M. N. Khan and L. H. Choudhury, RSC Adv., 2013, 3, 15576 RSC;
(f) Q. Yin and S.-L. You, Org. Lett., 2013, 15, 4266 CrossRef CAS PubMed.
-
(a) X. Zhang, C. Yang, D. Z. Negrerie and Y. Du, Chem.–Eur. J., 2015, 21, 5193 CrossRef CAS PubMed;
(b) R. M. Letcher, N. C. Kwok, W. H. Lo and K. W. Ng, J. Chem. Soc., Perkin Trans. 1, 1998, 1715 RSC;
(c) J. L. Bullington and J. H. Dodd, J. Heterocycl. Chem., 1998, 35, 397 CrossRef CAS.
-
(a) D. Z. Yang, P. Wang, J. Z. Liu, H. L. Xing, Y. Liu, W. C. Xie and G. S. Zhao, Bioorg. Med. Chem., 2014, 22, 1850 CrossRef PubMed;
(b) J. A. Sindac, S. J. Barraza, C. J. Dobry, J. Xiang, P. K. Blakely, D. N. Irani, R. F. Keep, D. J. Miller and S. D. Larsen, J. Med. Chem., 2013, 56, 9222 CrossRef CAS PubMed;
(c) L. D. Jennings, K. W. Foreman, T. S. Rush III, D. H. H. Tsao, L. Mosyak, S. L. Kincaid, M. N. Sukhdeo, A. G. Sutherland, W. Ding, C. H. Kenny, C. L. Sabus, H. Liu, E. G. Dushin, S. L. Moghazeh, P. Labthavikul, P. J. Petersen, M. Tuckman and A. V. Ruzin, Bioorg. Med. Chem., 2004, 12, 5115 CrossRef CAS PubMed;
(d) H. Matter, E. Defossa, U. Heinelt, P.-M. Blohm, D. Schneider, A. Muller, S. Herok, H. Schreuder, A. Liesum, V. Brachvogel, P. Lonze, A. Walser, F. Al-Obeidi and P. Wildgoose, J. Med. Chem., 2002, 45, 2749 CrossRef CAS PubMed;
(e) G. Tarzia, G. Diamantini, B. D. Giacomo and G. Spadoni, J. Med. Chem., 1997, 40, 2003 CrossRef CAS PubMed.
-
(a) C. B. Lavery, R. McDonald and M. Stradiotto, Chem. Commun., 2012, 48, 7277–7279 RSC;
(b) J. Barluenga, A. Jiménez-Aquino, C. Valdés and F. Aznar, Angew. Chem., Int. Ed., 2007, 46, 1529–1532 CrossRef CAS PubMed;
(c) Y.-Q. Fang and M. Lautens, J. Org. Chem., 2008, 73, 538–549 CrossRef CAS PubMed;
(d) L. Ackermann, Org. Lett., 2005, 7, 439–442 CrossRef CAS PubMed.
-
(a) R. Dalpozzo, Chem. Soc. Rev., 2015, 44, 742 RSC;
(b) H. Wu, Y. P. He and F. Shi, Synthesis, 2015, 47, 1990 CrossRef CAS;
(c) Y. C. Zhang, J. J. Zhao, F. Jiang, S. B. Sun and F. shi, Angew. Chem., Int. Ed., 2014, 53, 13912 CrossRef CAS PubMed;
(d) J. J. Zhao, S. B. Sun, S. H. He, Q. Wu and F. shi, Angew. Chem., Int. Ed., 2015, 54, 5460 CrossRef CAS PubMed;
(e) S. Dhiman and S. S. V. Ramasastry, Chem. Commun., 2015, 557 RSC;
(f) Y. M. Wang, H. H. Zhang, C. Li, T. Fan and F. Shi, Chem. Commun., 2016, 1804 RSC;
(g) R. Kancherla, T. Naveen and D. Maiti, Chem.–Eur. J., 2015, 21, 8723 CrossRef CAS PubMed;
(h) U. Sharma, R. Kancherla, T. Naveen, S. Agasti and D. Maiti, Angew. Chem., Int. Ed., 2014, 53, 11895 CrossRef CAS PubMed.
-
(a) L. T. Kaspar and L. Ackermann, Tetrahedron, 2005, 61, 11311–11316 CrossRef CAS;
(b) Y. H. Zhang, J. P. Donahue and C.-J. Li, Org. Lett., 2007, 9, 627 CrossRef CAS PubMed;
(c) R. Vicente, Org. Biomol. Chem., 2011, 6469 RSC.
-
(a) Z. Gao, C. Wang, C. Yuan, L. Zhou, Y. Xiao and H. Guo, Chem. Commun., 2015, 51, 12653 RSC;
(b) M. Yang, J. Tang and R. Fan, Chem. Commun., 2012, 48, 11775 RSC;
(c) Y. Oda, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2012, 14, 664 CrossRef CAS PubMed;
(d) J. Gao, Y. Shao, J. Zhu, J. Zhu, H. Mao, X. Wang and X. Lv, J. Org. Chem., 2014, 79, 9000 CrossRef CAS PubMed.
-
(a) V. Kavala, C.-C. Wang, D. K. Barange, C.-W. Kuo, P.-M. Lei and C.-F. Yao, J. Org. Chem., 2012, 77, 5022 CrossRef CAS PubMed;
(b) V. Kavala, D. Janreddy, M. J. Raihan, C.-W. Kuo, C. Ramesh and C.-F. Yao, Adv. Synth. Catal., 2012, 354, 2229 CrossRef CAS;
(c) T. Kotipalli, V. Kavala, D. Janreddy, C.-W. Kuo, T.-S. Kuo, H.-N. Huang, C.-H. He and C.-F. Yao, RSC Adv., 2014, 4, 2274 RSC;
(d) V. Kavala, C.-C. Wang, Y.-H. Wang, C.-W. Kuo, D. Janreddy, W.-C. Huang, T.-S. Kuo, C.-H. He, M.-L. Chen and C.-F. Yao, Adv. Synth. Catal., 2014, 356, 2609 CrossRef CAS;
(e) S. D. Gawande, V. Kavala, M. R. Zanwar, C.-W. Kuo, W.-C. Huang, T.-S. Kuo, H.-N. Huang, C.-H. He and C.-F. Yao, Adv. Synth. Catal., 2014, 356, 2599 CrossRef CAS;
(f) S. D. Gawande, M. R. Zanwar, V. Kavala, C.-W. Kuo, R. R. Rajawinslin and C.-F. Yao, Adv. Synth. Catal., 2015, 357, 168 CrossRef CAS;
(g) T. Kotipalli, D. Janreddy, V. Kavala, V. Bandi, C.-W. Kuo and C.-F. Yao, Eur. J. Org. Chem., 2016, 1182 CrossRef CAS.
-
(a) T. Songsichan, J. Promsuk, V. Rukachaisirikul and J. Kaeobamrung, Org. Biomol. Chem., 2014, 12, 4571 RSC;
(b) W. Xu, Y. Jin, H. Liu, Y. Jiang and H. Fu, Org. Lett., 2011, 13, 1274 CrossRef CAS PubMed;
(c) D. Chen, Q. Chen, M. Liu, S. Dai, L. Huang, J. Yang and W. Bao, Tetrahedron, 2013, 69, 6461 CrossRef CAS;
(d) D. Miao, X. Shi, G. He, Y. Tong, Z. Jiang and S. Han, Tetrahedron, 2015, 71, 431 CrossRef CAS;
(e) H. Tian, H. Qiao, C. Zhua and H. Fu, RSC Adv., 2014, 4, 2694 RSC;
(f) T. Li, M. Chen, L. Yang, Z. Xiong, Y. Wang, F. Li and D. Chen, Tetrahedron, 2016, 72, 868 CrossRef CAS.
-
(a) L. Li, M. Wang, X. Zhang, Y. Jiang and D.-W. Ma, Org. Lett., 2009, 11, 1309 CrossRef CAS PubMed;
(b) J. Pan, Z. Xu, R. Zeng and J. Zou, Chin. J. Chem., 2013, 31, 1022 CrossRef CAS;
(c) S. Sarkar, R. Pal and A. K. Sen, Tetrahedron Lett., 2013, 54, 4273 CrossRef CAS;
(d) S. Sarkar, S. Dutta, R. Dey and S. Naskar, Tetrahedron Lett., 2012, 53, 6789 CrossRef CAS;
(e) A. Gogoi, S. Guin, S. K. Rout, G. Majji and B. K. Patel, RSC Adv., 2014, 4, 59902 RSC;
(f) F. M. Irudayanathan, J. Noh, J. Choi and S. Lee, Adv. Synth. Catal., 2014, 356, 3433 CrossRef CAS;
(g) J. Dong, F. Wang and J. You, Org. Lett., 2014, 16, 2884 CrossRef CAS PubMed;
(h) S. B. Munoz, A. N. Aloia, A. K. Moore, A. Papp, T. Mathew, S. Fustero, G. A. Olah and G. K. Surya Prakash, Org. Biomol. Chem., 2016, 14, 85 RSC;
(i) J. Pan, Z. Xu, R. Zeng and J. Zou, Chin. J. Chem., 2013, 31, 1022 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1484056–1484061. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra15002k |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diastereoselectivity ratio.
2 diastereoselectivity ratio.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9
1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1
2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2
1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.9
2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d
1d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) on silica gel to afford desired product.
4) on silica gel to afford desired product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) on silica gel to afford desired product.
4) on silica gel to afford desired product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on silica gel to afford desired product.
1) on silica gel to afford desired product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether/ethyl acetate, 10
petroleum ether/ethyl acetate, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) on silica gel to afford desired product.
3) on silica gel to afford desired product.