Post polymer modification of polyethylenimine with citrate esters: selectivity and hydrophobicity†
Justine Waggel and
Robert T. Mathers*
Department of Chemistry, The Pennsylvania State University, New Kensington, Pennsylvania 15068, USA. E-mail: rtm11@psu.edu
First published on 22nd June 2016
Abstract
Citrate esters selectively react with primary (1°) amines on branched polyethylenimine (PEI). As the functionalization occurred, the water soluble PEI transformed into a thermoset. Adjusting stoichiometry and type of citrate ester allowed fine-tuning of hydrophobicity, modulus, and swelling behavior. Based on control experiments of citrate esters with model amines, FTIR analysis of thermosets, swelling experiments, and TGA data, molecular models were constructed to represent the thermosets. Quantifying the hydrophobicity of these molecular models with computational octanol–water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct) partition coefficients provided a method to assess how hydrophobicity changes during a post polymer modification (PPM) reaction.
Poct) partition coefficients provided a method to assess how hydrophobicity changes during a post polymer modification (PPM) reaction.
Introduction
Although most areas of science have unique terminology to describe interesting phenomena within sub-fields, the terms hydrophobic and hydrophilic cut across many disciplines. While these terms provide a great framework for understanding and explaining certain experimental observations, a more quantitative approach is frequently desired.1,2Given universal interest in “hydrophobic” surfaces,2,3 monomers,4–6 micelles,7 polymers,8,9 peptides,10 proteins,11 molecules,12 solvents,13 and drugs,14 comparing one system to another is often necessary. While most researchers have developed an intuition about hydrophobicity, a great deal of experimental2,15–18 and computational19–21 effort has been done to quantify the qualitative nature of hydrophobic systems. In particular, HPLC assessment of hydrophobicity has been insightful for monomers16 and star polymers.22
Recently, our efforts to predict hydrophobicity of polymers utilized octanol–water partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct).23 This method received inspiration from medicinal chemists who use computational log
Poct).23 This method received inspiration from medicinal chemists who use computational log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values to describe the hydrophobicity of drug-like molecules.14,24 Since monomers and drug-like molecules share many functional groups, log
Poct values to describe the hydrophobicity of drug-like molecules.14,24 Since monomers and drug-like molecules share many functional groups, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values provide a convenient method for assessing hydrophobicity. To take into account the changes that monomers undergo during a polymerization, log
Poct values provide a convenient method for assessing hydrophobicity. To take into account the changes that monomers undergo during a polymerization, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values for oligomeric sequences of monomers helped quantify hydrophobicity of the corresponding polymer. As such, negative values indicated water solubility while positive values suggested hydrophobic polymers.
Poct values for oligomeric sequences of monomers helped quantify hydrophobicity of the corresponding polymer. As such, negative values indicated water solubility while positive values suggested hydrophobic polymers.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values have an underutilized potential for quantifying hydrophobicity and could be very beneficial for post polymer modification (PPM) reactions. In recent years, attaching functional groups via a PPM strategy has received enormous attention.25–27 A few of the many benefits of PPM include improving biocompatibility,28 facilitating surface grafting,29 and providing access to various polymer architectures.30,31
Poct values have an underutilized potential for quantifying hydrophobicity and could be very beneficial for post polymer modification (PPM) reactions. In recent years, attaching functional groups via a PPM strategy has received enormous attention.25–27 A few of the many benefits of PPM include improving biocompatibility,28 facilitating surface grafting,29 and providing access to various polymer architectures.30,31
To illustrate the concept of quantifying hydrophobicity during PPM reactions, we identified several citrate esters with a range of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values and envisioned the covalent attachment to a polymer. Our interest in citrate esters developed during use of citric acid as a monomer32 and interest in non-toxic molecules.33,34 Although the benign nature of citrate esters makes them very suitable as plasticizers for PLA35 and cellulose acetate,36 they are rarely employed as monomers or crosslinkers.
Poct values and envisioned the covalent attachment to a polymer. Our interest in citrate esters developed during use of citric acid as a monomer32 and interest in non-toxic molecules.33,34 Although the benign nature of citrate esters makes them very suitable as plasticizers for PLA35 and cellulose acetate,36 they are rarely employed as monomers or crosslinkers.
Polyethylenimine (PEI) has amines that could potentially react with a variety of functional groups. For instance, PEI has been reacted with acid chlorides,37 anhydrides,38,39 aldehydes,40 alkyl halides,37 carbonates,41 carboxylic acids,42 and methacrylates.43 Many of these hydrophobic modifications improve electrospinning, gene transfection, and alter LCST behavior. However, these studies are representative of the widespread empirical efforts and approaches to addressing hydrophobicity. At present, the reaction of PEI with citrate esters has not been reported and provides an opportunity to investigate and quantify hydrophobicity with partition coefficients.
Experimental
Materials
Triethyl citrate (TEC, >98%, Sigma Aldrich), tributyl citrate (TBC, ≥97.0%, Sigma Aldrich), triethyl 2-acetylcitrate (TEC-OAc, 99%, Sigma Aldrich), tributyl O-acetylcitrate (TBC-OAc, 98%, Sigma Aldrich), allyl amine (98+%, Acros Organics), benzyl amine (99%, Acros Organics), n-butylamine (99%, Acros Organics), diethyl amine (>99.5%, Sigma Aldrich), di-n-butylamine (99%, Acros Organics), diisopropyl amine (99+%, Acros Organics), diisopropylethyl amine (99.5+%, Acros Organics), N,N-dimethylformamide (DMF, 99.8%, anhydrous, Sigma Aldrich) hexamethyleneimine (99%, Acros Organics), hexanes (HPLC grade, Fisher), morpholine (99+%, Acros Organics), oleyl amine (technical grade, primary amine content > 98%), n-tripropyl amine (98%, Acros Organics), N,N,N-trimethylethylene diamine (98%, Acros Organics), poly(ethyleneimine) (PEI) (Mn ∼ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and Mn ∼ 600 g mol−1, Sigma Aldrich), and water (HPLC grade, Sigma Aldrich) were used as received.
000 g mol−1 and Mn ∼ 600 g mol−1, Sigma Aldrich), and water (HPLC grade, Sigma Aldrich) were used as received.
Characterization
FTIR spectra were recorded with a diamond ATR crystal on a ThermoFisher Nicolet iS10 FTIR spectrometer. FTIR spectra were averaged from 64 scans at a 4 cm−1 resolution. The thermal stability and decomposition temperature (Td) was analysed with a TA Instruments thermogravimetric analyzer (TGA) Q500 at 20 °C min−1 under nitrogen.Young's modulus and elongation at break was obtained by Dynamic Mechanical Analysis (DMA) using a TA Instruments Q800. Samples were ∼3–4 mm wide, ∼1 mm thick and ∼25 mm long. After attaching the sample to a film clamp, data was collected at ambient temperature and 1 N min−1. The Young's modulus was calculated from the slope of stress versus strain graphs between 0 and 2% strain. The reported values for modulus value and elongation at break were determined by averaging results from four samples.
Swelling studies were conducted with water or hexanes at ambient temperature. Samples (∼200–240 mg) were cut (∼1 mm × ∼4 mm × ∼25 mm) and immersed in water. The mass increase was measured gravimetrically over a 24 h period. The swelling ratio (SR) was calculated from the following equation: SR (%) = [[(mass of swelled polymer) − (mass of dry polymer)]/(mass of dry polymer)] × 100.
Polymerization method
All polymerizations were heated in air using aluminium pans (Fisher Scientific, volume ∼ 70 mL). The stoichiometry was adjusted to produce polymer films with diameter of ∼6.5 cm and thickness of ∼1 mm.General procedure for kinetic experiments in Fig. 1
PEI (Mn ∼ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) (2.000 g, ∼11.6 mmol 1° NH2 groups) was mixed with citrate ester (1.45 mmol) ([1° NH2]/[TEC] = 8) in an aluminum pan while heating at 60 °C for several minutes. After mixing, the film was reacted at 80–100 °C on a temperature controlled hotplate and periodically monitored by FTIR spectroscopy.
000 g mol−1) (2.000 g, ∼11.6 mmol 1° NH2 groups) was mixed with citrate ester (1.45 mmol) ([1° NH2]/[TEC] = 8) in an aluminum pan while heating at 60 °C for several minutes. After mixing, the film was reacted at 80–100 °C on a temperature controlled hotplate and periodically monitored by FTIR spectroscopy.
Reaction of PEI and TEC for DMA and swelling experiments
PEI (Mn ∼ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) (2.000 g, ∼11.6 mmol 1° NH2 groups) was mixed with TEC (1.613 g, 5.8 mmol, [1° NH2]/[TEC] = 2) in an aluminum pan while heating at 60 °C for several minutes. After mixing, the film was reacted at 100 °C on a temperature controlled hotplate. After 2 h, the mass loss via evaporation of ethanol was 0.327 g.
000 g mol−1) (2.000 g, ∼11.6 mmol 1° NH2 groups) was mixed with TEC (1.613 g, 5.8 mmol, [1° NH2]/[TEC] = 2) in an aluminum pan while heating at 60 °C for several minutes. After mixing, the film was reacted at 100 °C on a temperature controlled hotplate. After 2 h, the mass loss via evaporation of ethanol was 0.327 g.
Reaction of PEI and TBC for DMA and swelling experiments
PEI (Mn ∼ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) (2.000 g, ∼11.6 mmol 1° NH2 groups) was mixed with TBC (2.09 g, 5.8 mmol, [1° NH2]/[TBC] = 2) in an aluminum pan while heating at 60 °C for several minutes. After mixing, the film was reacted at 100 °C on a temperature controlled hotplate. After 2 h, the mass loss via evaporation of butanol was 0.382 g.
000 g mol−1) (2.000 g, ∼11.6 mmol 1° NH2 groups) was mixed with TBC (2.09 g, 5.8 mmol, [1° NH2]/[TBC] = 2) in an aluminum pan while heating at 60 °C for several minutes. After mixing, the film was reacted at 100 °C on a temperature controlled hotplate. After 2 h, the mass loss via evaporation of butanol was 0.382 g.
General procedure with TBC-OAc
PEI (Mn ∼ 600 g mol−1) (2.000 g, ∼11.6 mmol 1° NH2 groups) was mixed with TBC-OAc (1.167 g, 2.9 mmol) at 60 °C. After 30 minutes, the temperature was increased to 100 °C on a temperature controlled hotplate. After 3 h at 100 °C, film formation was noted. The reaction continued for 24 h to promote evaporation of butanol. Then, the film was cooled to ambient temperature and analysed by FTIR spectroscopy (Absamide/Absester = 3.2). The SR after 2 h in water was 329%.Control experiments with amines
In a vial (4 mL), citrate ester (TEC or TBC) (0.2 mL) and a small molecule amine (0.3 mL) (Table S1†) were combined at ambient temperature. The vial was capped and heated for 2 h at 80 °C while stirring. Then, the reaction was cooled to ambient temperature and analysed by FTIR spectroscopy.Hydrophobicity calculations
Molecular models were constructed using Chem3D Pro version 13.0.2.3021 and Materials Studio 7.0. After building each structure, the model was minimized using the MM2 forcefield (Chem3D) and geometry optimization in Forcite module (Materials Studio). The Forcite module used a Universal forcefield and Smart algorithm. Afterwards, the partition coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct for Chem3D and A
Poct for Chem3D and A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P for Materials Studio) were extracted from the chemical properties module. The Connolly molecular surface area was calculated using a probe of 1.4 Å.
P for Materials Studio) were extracted from the chemical properties module. The Connolly molecular surface area was calculated using a probe of 1.4 Å.
Results and discussion
Structure and reactivity of PEI
The ring opening polymerization of aziridine produces branched PEI (Scheme 1) with primary (1°), secondary (2°), and tertiary (3°) amine groups.44 Although the theoretical ratio of 1°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2°
2°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3° amines has been reported to be 1
3° amines has been reported to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, some variability exists depending on synthetic procedures.45
1, some variability exists depending on synthetic procedures.45
Since hexamethylene diamine [H2N(CH2)6NH2] reacts with triethyl citrate,46 we hypothesized that the 1° amines on PEI would also be reactive towards commercially available citrate esters. Indeed, based on FTIR spectroscopy (Fig. S1†), amines on PEI reacted with citrate esters in Scheme 2 and converted the esters to amides via nucleophilic addition. Reactions of PEI and citrate esters were also accompanied by mass loss due to evaporation of ethanol or butanol.
Furthermore, experiments with TEC, TEC-OAc, and TBC mixed well with PEI (Mn ∼ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and provided homogeneous films without the need for organic solvents. In contrast, the larger log
000 g mol−1) and provided homogeneous films without the need for organic solvents. In contrast, the larger log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct value for TBC-OAc resulted in mixing and phase separation issues. Reducing the molecular weight of PEI to Mn ∼ 600 g mol−1 or adding a small quantity of DMF improved homogeneity. The large disparity in hydrophobicity between PEI and TBC-OAc highlights a challenge that exists for hydrophobic modification of water soluble polymers.
Poct value for TBC-OAc resulted in mixing and phase separation issues. Reducing the molecular weight of PEI to Mn ∼ 600 g mol−1 or adding a small quantity of DMF improved homogeneity. The large disparity in hydrophobicity between PEI and TBC-OAc highlights a challenge that exists for hydrophobic modification of water soluble polymers.
Kinetics
After determining the feasibility of reacting PEI with citrate esters in Scheme 2, FTIR spectroscopy (Fig. 1) confirmed that a range of temperatures allowed the ester groups in citrate esters to react with PEI. In fact, these polymerizations underwent gelation after ∼10 min at 100 °C and ∼20 min at 80 °C.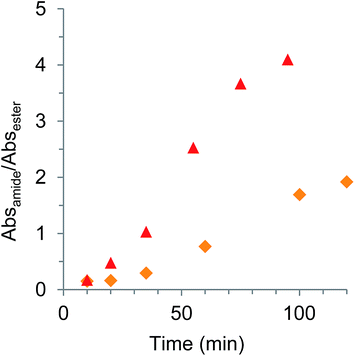 | ||
Fig. 1 FTIR spectroscopy data for reaction of PEI (Mn = 10k) and TEC at 80 °C ( ) and 100 °C ( ) and 100 °C ( ). Absamide/Absester ratio calculated from height of amide (1662 cm−1) and ester (1734 cm−1) absorbances. ). Absamide/Absester ratio calculated from height of amide (1662 cm−1) and ester (1734 cm−1) absorbances. | ||
In Fig. 1, the formation of the amide groups occurred with simultaneous decrease in the ester absorbance for TEC. At moderate conversions, the amide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ester ratio gave predictable and reproducible results for a constant film thickness. Based on regression analysis of FTIR data (Fig. S2†), the larger butyl groups on TBC lowered the reactivity (krel = 1) compared to the less sterically hindered TEC (krel = 1.8).
ester ratio gave predictable and reproducible results for a constant film thickness. Based on regression analysis of FTIR data (Fig. S2†), the larger butyl groups on TBC lowered the reactivity (krel = 1) compared to the less sterically hindered TEC (krel = 1.8).
Selectivity of primary amines
While analysis of Fig. 1 indicated formation of amide bonds, further experiments were necessary to determine reactivity differences between 1°, 2°, and 3° amines in PEI (Scheme 1).After conducting control experiments (Table S1†) with a large number of 1°, 2°, and 3° amines to simulate the chemical environment of PEI, FTIR spectroscopy (Fig. S3 and S4†) confirmed citrate esters selectively react with primary amines. Generally, 1° amines were probably at least two orders of magnitude more reactive than 2° amines. For instance, 2° amines, like diethyl amine and dibutyl amine, did not react with TEC and produce detectable amide absorbances (Fig. 2) after 2 h at 80 °C or 100 °C. Dibutyl amine was also unreactive with TBC under similar conditions. In contrast, 1° amines reacted without difficulty under similar conditions and gave a reactivity profile that decreased as follows: butyl > allyl > benzyl. A very small amount of reactivity occurred with cyclic secondary amines (i.e. morpholine and hexamethyleneimine). However, compared to butyl amine, the magnitude of this reactivity was insignificant. Tertiary amines, like diisopropylethyl amine and n-tripropyl amine were also unreactive.
 | ||
| Fig. 2 FTIR spectra for the product of TEC and butyl amine (solid line) after 2 h at 80 °C compared to dibutyl amine (dashed line) and TEC after 2 h at 100 °C. | ||
Often, the focus of PPM reactions with amines or thiols involves increasing the reactivity of activated esters to obtain quantitative yield47,48 or selectively preparing block copolymers.49 Surprisingly, PPM reactions that allow 1° amines to selectivity react with esters, like TEC and TBC, have not been reported. Interestingly, secondary amines will react with activated esters,50 epoxides,51 urethanes52 dithiocarbonates,53 and acrylates,54,55 but do not react with carbonates.51 Since 2° amines are very unlikely to react with citrate esters, the observed selectively benefits the goal of obtaining hydrophobic thermosets and allows the existence of terminal units in Scheme 3.
 | ||
| Scheme 3 Representation of terminal and crosslinked citrate esters (R = ethyl, butyl; R′ = H, acetate). | ||
Based on control experiments, Scheme 3 depicts several amide bonds that result from the reaction of 1° amines and citrate esters. Since the structure in Scheme 3 formed the basis for constructing computational models (Schemes S1–S7†) to quantify hydrophobicity, a combination of reaction kinetics (Fig. 1), control experiments with model amines (Fig. 2), thermal analysis, and swelling studies provided a synergistic perspective. Depending on experimental parameters, both terminal and crosslinked citrate groups are expected.
Thermal analysis
As the 1° amines selectively react with citrate esters, the thermoplastic PEI becomes functionalized with amide groups and begins to crosslink into a thermoset (Scheme 3). During the functionalization reaction, the formation of each amide group produces an alcohol (i.e. ethanol or butanol) that will potentially evaporate given appropriate time and temperature.To investigate the propensity for crosslinking and evaporation of ethanol or butanol, a series of polymerizations were monitored by TGA. Based on the onset of decomposition (∼340–370 °C) from weight loss profiles, crosslinking increased with increasing time (Fig. S5†) and temperature (Fig. S6†). Additionally, reactivity of the citrate ester played a role and TEC gave more crosslinking than TBC after 1 h at 100 °C (Fig. S7†). This observation that TEC is more reactive than TBC correlates with FTIR analysis (Fig. S2†). Considering the mass loss below 300 °C, TGA data indicates short reaction times (i.e. < 2 h) and lower reaction temperatures (i.e. < 100 °C) produced films that contained alcohol as well as unreacted citrate ester.
Mechanical properties
To further expand upon the perspective established by TGA and FTIR spectroscopy, a series of flexible films were investigated by DMA. After reacting PEI and citrate esters for 2 h at 100 °C, the Young's Modulus (YM) value for [1° NH2]/[TEC] = 8 was 10.6 ± 0.34 MPa. When changing the [1° NH2]/[TEC] ratio from 8 to 4, larger elongation at break (Fig. S8†) and higher YM values (Fig. S9†) were observed relative to TBC.Hydrophobicity calculations
In order to determine the effect of attaching citrate esters to PEI, a series of molecular models replicated the local environment of PEI thermosets. The models, which were based on FTIR and TGA data, considered scenarios without crosslinks (Scheme S1†) and with crosslinks (Schemes S2–S7†). As shown in Fig. 3 and S10,† these oligomeric models allowed a theoretical calculation of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values for various [1° amine]/[citrate ester] ratios. In fact, the range of stoichiometry in Fig. 3 provided an excess of 1° amines as well as an excess of esters. Previously, solvatochromatic dye experiments with Nile Red validated calculation of hydrophobicity for homopolymers and copolymers using log
Poct values for various [1° amine]/[citrate ester] ratios. In fact, the range of stoichiometry in Fig. 3 provided an excess of 1° amines as well as an excess of esters. Previously, solvatochromatic dye experiments with Nile Red validated calculation of hydrophobicity for homopolymers and copolymers using log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values.23 To facilitate comparison between different sized citrate esters, the log
Poct values.23 To facilitate comparison between different sized citrate esters, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values were normalized with surface area (SA).
Poct values were normalized with surface area (SA).
 | ||
Fig. 3 Hydrophobicity calculations of PEI oligomers based on octanol–water partition coefficients. The data represents the influence of terminal citrate esters on PEI without crosslinks using TEC ( ), TBC ( ), TBC ( ), and TBC-OAc ( ), and TBC-OAc ( ) groups. See Tables S2 and S3† for additional information and data. The solid lines represent logarithmic regression. ) groups. See Tables S2 and S3† for additional information and data. The solid lines represent logarithmic regression. | ||
In regards to Fig. 3, several observations are worth mentioning. First, the amount of hydrophobicity is initially small for an excess of 1° amines. As reflected by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA values, attachment of citrate esters to 1° amines during PPM increases hydrophobicity. For a given conversion, the theoretical increase in hydrophobicity for terminal citrate esters would decrease as follows: TBC-OAc > TBC > TEC. Second, if all 1° amine groups were reacted with TEC or TBC groups, the hydrophilic nature of PEI would still result in negative log
Poct/SA values, attachment of citrate esters to 1° amines during PPM increases hydrophobicity. For a given conversion, the theoretical increase in hydrophobicity for terminal citrate esters would decrease as follows: TBC-OAc > TBC > TEC. Second, if all 1° amine groups were reacted with TEC or TBC groups, the hydrophilic nature of PEI would still result in negative log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA values. Consequently, these materials with TEC or TBC groups will be expected to swell in water. Third, terminal citrate groups, which only have 1 ester replaced with an amide group, would contribute a larger increase in hydrophobicity than crosslinked groups. For example, the addition of crosslinking to molecular models (Schemes S2–S7†) lowers the number of alkyl esters. Consequently, slightly lower log
Poct/SA values. Consequently, these materials with TEC or TBC groups will be expected to swell in water. Third, terminal citrate groups, which only have 1 ester replaced with an amide group, would contribute a larger increase in hydrophobicity than crosslinked groups. For example, the addition of crosslinking to molecular models (Schemes S2–S7†) lowers the number of alkyl esters. Consequently, slightly lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA values (Fig. S10†) were observed compared to models (Scheme S1†) without crosslinking (Fig. 3).
Poct/SA values (Fig. S10†) were observed compared to models (Scheme S1†) without crosslinking (Fig. 3).
Swelling studies
Although branched PEI is a viscous polymer that easily dissolves in water at room temperature, the reaction with trifunctional citrate esters resulted in crosslinked films that swelled in water (Fig. 4). Since these films did not swell in hexanes, the negative log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct/SA values in Fig. 3 are very reasonable. Additionally, Fig. S11† suggests a relationship between the amount of alkyl esters and the swelling ratio.
Poct/SA values in Fig. 3 are very reasonable. Additionally, Fig. S11† suggests a relationship between the amount of alkyl esters and the swelling ratio.
Conclusion
The reaction of water soluble PEI with citrate esters appeared quite facile and occurred with a variety of alkyl substituted citrate esters. Based on FTIR spectroscopy, model reactions with 1°, 2°, and 3° amines confirmed the 1° amines on branched PEI preferentially react with citrate esters. This selectivity of 1° amines with citrate esters provided an avenue for thermosets with tunable hydrophobicity, modulus, and thermal stability.Computational log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values and experimental swelling data confirm PPM reactions between PEI and citrates esters experience a dramatic increase in hydrophobicity as the conversion increases. This change in hydrophobicity during PPM reactions would be expected to occur in other systems when a disparity exists between the hydrophobicity of a polymer and the functional group.
Poct values and experimental swelling data confirm PPM reactions between PEI and citrates esters experience a dramatic increase in hydrophobicity as the conversion increases. This change in hydrophobicity during PPM reactions would be expected to occur in other systems when a disparity exists between the hydrophobicity of a polymer and the functional group.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values provided a less empirical roadmap for designing and conducting synthetic experiments. In addition, this approach offers clear direction for obtaining certain amounts of hydrophobicity and may reduce the need for exhaustive experimentation. As such, the concept of creating polymer libraries as well as hydrophobically modified water-soluble polymers based on acrylamides,56 oxazolines,57 acrylates,58 and aliphatic amines59 could benefit from this strategy with log
Poct values provided a less empirical roadmap for designing and conducting synthetic experiments. In addition, this approach offers clear direction for obtaining certain amounts of hydrophobicity and may reduce the need for exhaustive experimentation. As such, the concept of creating polymer libraries as well as hydrophobically modified water-soluble polymers based on acrylamides,56 oxazolines,57 acrylates,58 and aliphatic amines59 could benefit from this strategy with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Poct values.
Poct values.
Acknowledgements
R. T. M. thanks the National Science Foundation (CHE-MSN and DMR-Polymers) for support under Grant CHE 1308247.Notes and references
- E. A. Vogler, in Water in Biomaterials Surface Science, ed. M. Morra, John Wiley, 2001 Search PubMed.
- L. Gao, T. J. McCarthy and X. Zhang, Langmuir, 2009, 25, 14100–14104 CrossRef CAS PubMed.
- R. P. Quirk, R. T. Mathers, T. Cregger and M. D. Foster, Macromolecules, 2002, 35, 9964–9974 CrossRef CAS.
- D. Dakshinamoorthy, S. P. Lewis, M. P. Cavazza, A. M. Hoover, D. F. Iwig, K. Damodaran and R. T. Mathers, Green Chem., 2014, 16, 1774–1783 RSC.
- D. Dakshinamoorthy, A. K. Weinstock, K. Damodaran, D. F. Iwig and R. T. Mathers, ChemSusChem, 2014, 7, 2923–2929 CrossRef CAS PubMed.
- J. M. Delancey, M. D. Cavazza, M. G. Rendos, C. J. Ulisse, S. G. Palumbo and R. T. Mathers, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3719–3727 CrossRef CAS.
- W.-M. Wan, P. D. Pickett, D. A. Savin and C. L. McCormick, Polym. Chem., 2014, 5, 819–827 RSC.
- M. H. Park, M. K. Joo, B. G. Choi and B. Jeong, Acc. Chem. Res., 2012, 45, 424–433 CrossRef CAS PubMed.
- S. H. Lahasky, L. Lu, W. A. Huberty, J. Cao, L. Guo, J. C. Garno and D. Zhang, Polym. Chem., 2014, 5, 1418–1426 RSC.
- A. Som, A. O. Tezgel, G. J. Gabriel and G. N. Tew, Angew. Chem., Int. Ed., 2011, 50, 6147–6150 CrossRef CAS PubMed.
- C. H. Robert and B. M. Pettitt, J. Phys.: Condens. Matter, 2016, 28, 083003 CrossRef PubMed.
- X. Huang, C. J. Margulis and B. J. Berne, J. Phys. Chem. B, 2003, 107, 11742–11748 CrossRef CAS.
- P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245–1259 RSC.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3–25 CrossRef CAS.
- E. I. Vargha-Butler, E. Kiss, C. N. C. Lam, Z. Keresztes, E. Kalman, L. Zhang and A. W. Neumann, Colloid Polym. Sci., 2001, 279, 1160–1168 Search PubMed.
- A. Som, A. Reuter and G. N. Tew, Angew. Chem., Int. Ed., 2012, 51, 980–983 CrossRef CAS PubMed.
- D. Kępińska, A. Budniak, K. Kijewska, G. J. Blanchard and M. Mazur, Polymer, 2013, 54, 4538–4544 CrossRef.
- M. Dąbrowska, Ł. Komsta, J. Krzek and K. Kokoszka, Biomed. Chromatogr., 2015, 29, 1759–1768 CrossRef PubMed.
- H. Liu, Y. Li, W. E. Krause, O. J. Rojas and M. A. Pasquinelli, J. Phys. Chem. B, 2012, 116, 1570–1578 CrossRef CAS PubMed.
- M. T. Nguyen, A. L. Chaffee, R. I. Boysen, D. V. Nicolau and M. T. W. Hearn, Mol. Simul., 2016, 42, 257–269 CrossRef CAS.
- A. Bar-Even, E. Noor, A. Flamholz, J. M. Buescher and R. Milo, PLoS Comput. Biol., 2011, 7, 1–7 CrossRef PubMed.
- M. Unverferth and M. A. R. Meier, Polymer, 2014, 55, 5571–5575 CrossRef CAS.
- A. J. D. Magenau, J. A. Richards, M. A. Pasquinelli, D. A. Savin and R. T. Mathers, Macromolecules, 2015, 48, 7230–7236 CrossRef CAS.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 2001, 46, 3–26 CrossRef CAS PubMed.
- A. Das and P. Theato, Chem. Rev., 2016, 116, 1434–1495 CrossRef CAS PubMed.
- K. A. Günay, P. Theato and H.-A. Klok, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1–28 CrossRef.
- S. Tempelaar, L. Mespouille, O. Coulembier, P. Dubois and A. P. Dove, Chem. Soc. Rev., 2013, 42, 1312–1336 RSC.
- D. Liang, B. S. Hsiao and B. Chu, Adv. Drug Delivery Rev., 2007, 59, 1392–1412 CrossRef CAS PubMed.
- C. J. Galvin and J. Genzer, Prog. Polym. Sci., 2012, 37, 871–906 CrossRef CAS.
- F. Moldenhauer and P. Theato, in Multi-Component and Sequential Reactions in Polymer Synthesis, ed. P. Theato, Springer, 2015, vol. 269, pp. 133–162 Search PubMed.
- E. Passaglia, S. Coiai and S. Augier, Prog. Polym. Sci., 2009, 34, 911–947 CrossRef CAS.
- J. Halpern, A. K. Weinstock, R. Urbanski, D. F. Iwig, R. T. Mathers and H. A. Von Recum, J. Biomed. Mater. Res., Part A, 2014, 102, 1467–1477 CrossRef PubMed.
- R. T. Mathers, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1–15 CrossRef CAS.
- K. Schroder, K. Matyjaszewski, K. J. T. Noonan and R. T. Mathers, Green Chem., 2014, 16, 1673–1686 RSC.
- N. Ljungberg and B. Wesslén, Polymer, 2003, 44, 7679–7688 CrossRef CAS.
- H.-M. Park, M. Misra, L. T. Drzal and A. K. Mohanty, Biomacromolecules, 2004, 5, 2281–2288 CrossRef CAS PubMed.
- Z. Liu, Z. Zhang, C. Zhou and Y. Jiao, Prog. Polym. Sci., 2010, 35, 1144–1162 CrossRef CAS.
- N. Khanam, C. Mikoryak, R. K. Draper and K. J. Balkus Jr, Acta Biomater., 2007, 3, 1050–1059 CrossRef CAS PubMed.
- C. Wang, X. Bao, X. Ding, Y. Ding, S. Abbad, Y. Wang, M. Li, Y. Su, W. Wang and J. Zhou, Polym. Chem., 2015, 6, 780–796 RSC.
- S. K. Tripathi, N. Gupta, M. Mahato, K. C. Gupta and P. Kumar, Colloids Surf., B, 2014, 115, 79–85 CrossRef CAS PubMed.
- P. Y. Teo, C. Yang, J. L. Hedrick, A. C. Engler, D. J. Coady, S. Ghaem-Maghami, A. J. T. George and Y. Y. Yang, Biomaterials, 2013, 34, 7971–7979 CrossRef CAS PubMed.
- D. Meneksedag-Erol, R. B. Kc, T. Tang and H. Uludağ, ACS Appl. Mater. Interfaces, 2015, 7, 24822–24832 Search PubMed.
- Y. Xia, J. Wang, S. Xu, Q. Liao, X. Zhu, Y. Wang and Y. Wang, J. Appl. Polym. Sci., 2013, 127, 3249–3255 CrossRef CAS.
- M.-M. Xun, J.-H. Zhang, Y.-H. Liu, J. Zhang, Y.-P. Xiao, Q. Guo, S. Li and X.-Q. Yu, RSC Adv., 2016, 6, 5391–5400 RSC.
- A. von Harpe, H. Petersen, Y. Li and T. Kissel, J. Controlled Release, 2000, 69, 309–322 CrossRef CAS PubMed.
- M. Fleischer, H. Blattmann and R. Mulhaupt, Green Chem., 2013, 15, 934–942 RSC.
- J.-M. Noy, M. Koldevitz and P. J. Roth, Polym. Chem., 2015, 6, 436–447 RSC.
- J. Lawrence and T. Emrick, ACS Appl. Mater. Interfaces, 2016, 8, 2393–2398 Search PubMed.
- Y. Li, H. T. T. Duong, M. W. Jones, J. S. Basuki, J. Hu, C. Boyer and T. P. Davis, ACS Macro Lett., 2013, 2, 912–917 CrossRef CAS.
- A. C. Engler, J. M. W. Chan, D. J. Coady, J. M. O'Brien, H. Sardon, A. Nelson, D. P. Sanders, Y. Y. Yang and J. L. Hedrick, Macromolecules, 2013, 46, 1283–1290 CrossRef CAS.
- Y. He, V. Goel, H. Keul and M. Möller, Macromol. Chem. Phys., 2010, 211, 2366–2381 CrossRef CAS.
- Q.-W. Lu, T. R. Hoye and C. W. Macosko, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 2310–2328 CrossRef CAS.
- B. Ochiai, J.-i. Nakayama, M. Mashiko, Y. Kaneko, T. Nagasawa and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5899–5905 CrossRef CAS.
- D. G. Anderson, C. A. Tweedie, N. Hossain, S. M. Navarro, D. M. Brey, K. J. Van Vliet, R. Langer and J. A. Burdick, Adv. Mater., 2006, 18, 2614–2618 CrossRef CAS.
- J. A. Redondo, R. Navarro, E. Martínez-Campos, M. Pérez-Perrino, R. París, J. L. López-Lacomba, C. Elvira, H. Reinecke and A. Gallardo, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2297–2305 CrossRef CAS.
- K. C. da Silveira, Q. Sheng, W. Tian, E. Fernandes Lucas and C. D. Wood, J. Appl. Polym. Sci., 2015, 132, 42797 CrossRef.
- R. Obeid, E. Maltseva, A. F. Thünemann, F. Tanaka and F. M. Winnik, Macromolecules, 2009, 42, 2204–2214 CrossRef CAS.
- T. M. Eggenhuisen, C. R. Becer, M. W. M. Fijten, R. Eckardt, R. Hoogenboom and U. S. Schubert, Macromolecules, 2008, 41, 5132–5140 CrossRef CAS.
- P. Ferruti, M. A. Marchisio and R. Duncan, Macromol. Rapid Commun., 2002, 23, 332–355 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14953g |
| This journal is © The Royal Society of Chemistry 2016 |