Evaluation of a tubular nano-composite ceramic membrane for hydrogen separation in methane steam reforming reaction
Mahdi Amanipour*a,
Ensieh Ganji Babakhani*b,
Jafar Towfighia and
Akbar Zamaniyanb
aChemical Engineering Department, Engineering Faculty, University of Tarbiat Modares, 14115-111, Tehran, Iran. E-mail: m.amanipour@modares.ac.ir; Fax: +98 21 8288 4180; Tel: +98 21 8288 3145
bGas Department, Research Institute of Petroleum Industry (RIPI), 14665-137, Tehran, Iran. E-mail: ganjie@ripi.ir; Fax: +98 21 44739716; Tel: +98 21 48252398
First published on 31st August 2016
Abstract
Tubular silica/alumina membranes with three different thicknesses of selective layer (75–100 nm) were successfully applied as membrane reactors for simultaneous production and purification of hydrogen. BET analysis indicated a decreasing trend in membrane characteristics by adding successive layers, which is well in agreement with the graded structure of the substrate. These membranes showed a high permeance of 5–11 × 10−7 mol m−2 s−1 Pa−1 and good separation factor of 20–300 at various temperatures (773–1073 K) in an equimolar mixture of H2, CH4, CO, and CO2. The reactor performance of the membranes was evaluated for the methane steam reforming (MSR) reaction – using a conventional nickel/alumina catalyst with 10 wt% of nickel on the alumina support – in the reaction temperature range of 773–1073 K and pressure range of 1–10 bar. X-ray diffraction measurements of the catalyst showed a good dispersion of nickel on the alumina substrate. The reactor results showed an increasing trend for H2 yield between 3.8–30 × 10−6 mol g−1 with both increasing temperature and pressure, but CH4 conversion decreased about 30% with increasing pressure from 1 to 10 bar. These results also indicate higher values of hydrogen yield and methane conversion in comparison to the equilibrium conditions for all membranes even up to 35% by increasing either temperature or pressure.
1. Introduction
Hydrogen has been nominated as a clean energy carrier and has attracted a great deal of attention in recent years.1,2 Hydrogen production on an industrial scale is mostly based on reforming of methane, while this hydrogen is mainly used in oil refineries, petrochemical plants, and as an energy source for use in fuel cell applications.3 The outlet stream of a steam reforming reactor consists of not only hydrogen, but also methane, carbon monoxide, carbon dioxide, vapor and some other components. Therefore, a separation unit is required to produce highly purified hydrogen. The main hydrogen separation and purification processes are cryogenic distillation, pressure swing adsorption, and membrane separation technologies.1 Membrane separation is a newly developed technique for gas purification, and has a number of advantages over conventional technologies of PSA and cryogenic distillation; such as high operational flexibility, low initial and operational cost, and low energy consumption.4,5Methane steam reforming (MSR) usually occurs at high temperature of 1000–1100 K (eqn (1)). Meanwhile, carbon monoxide generated from the reforming reaction reacts with water to produce hydrogen and carbon dioxide in reversible water-gas shift (WGS) reaction at 500–700 K (eqn (2)).6 There can also be another main reaction (eqn (3)) which is in fact a combination of the two reactions:
| CH4 + H2O ⇔ 3H2 + CO, ΔH = 225 kJ mol−1 at 1000 K | (1) |
| CO + H2O ⇔ H2 + CO2, ΔH = −191 kJ mol−1 at 1000 K | (2) |
| CH4 + 2H2O ⇔ 4H2 + CO2, ΔH = 34 kJ mol−1 at 1000 K | (3) |
As mentioned above, the process requires a high operating temperature because the overall reaction is endothermic. Such a high temperature results in some restrictions such as vast amount of energy consumption, expensive tubular reformers with huge burners, and irreversible coke formation in reactor. Therefore, a lower reaction temperature is practically and economically desirable. On the other hand, low temperature reforming cannot produce sufficient conversion for the actual industrial process. For example, when steam reforming of methane is carried out at 800 K and 2.0 MPa with steam/methane molar ratio of 3, the equilibrium conversion of methane is less than 20%.3
Membrane reactors have been used in order to reduce reaction temperature by separating hydrogen from reaction media and hence increasing the equilibrium conversion by shifting eqn (1) & (2) to the right side.7–9 Although a number of studies have been performed on membrane reactors for MSR reaction, most of them have focused attention on palladium-based membranes, which despite their high selectivity for hydrogen and low coke formation rates, suffer from low permeation flux and high capital investment at industrial scales.10–12 Ceramic membranes, and mostly amorphous silica membranes, are attractive substitutions because they are more economic and practical materials compared to any other inorganic membrane and can be used in steam reforming of methane to produce and separate hydrogen efficiently.13–22
Since separation of hydrogen is promoted by differential pressure of hydrogen between the reaction zone and the permeation side of the membrane reactor, it is preferred to keep the hydrogen pressure on the reaction zone as high as possible. However, hydrogen separation reduces the pressure; so a catalyst with high activity is needed to increase methane conversion that recovers hydrogen pressure to the new equilibrium situation.3 Hence, catalytic activity is an important factor in MSR reaction and many researchers have worked on preparation, characterization and loading of catalysts to enhance performance in reforming membrane reactors.23–26 However most of researches on MSR membrane reactor are performed in metal-type membrane reactors. These types of membranes usually suffer from low methane conversion because it is hard to reach a balance between reaction rates and hydrogen conversion through reaction media. Tong et al.24 have worked on steam reforming of methane in a Pd-based membrane reactor. The results showed good selectivity of hydrogen but the conversion was low because of low permeation due to dense palladium layer.
In this work, we focused our attention on performance evaluation of a new kind of silica/alumina nano-composite membrane which was synthesized in our laboratory previously.27,28 The aim of the experiments in here is to clearly identify the effect of membrane structural parameters and process conditions (pressure and temperature) on performance of these type of membranes. The membranes are mounted in an experimental membrane reactor module to produce and purify hydrogen in a methane steam reforming reaction and effect of temperature and pressure on methane conversion and hydrogen production was evaluated experimentally.
2. Experimental
Nano-composite ceramic membranes composed of a thin layer of SiO2/Al2O3 on top of alumina supports with a graded structure are applied in order to produce and separate hydrogen in an integrated membrane reactor. These membranes have been prepared and characterized by our group and have been reported previously.27,28 Briefly, synthesis procedure is as follows: 0.2 mol of aluminum tri-isopropilate was added to 300 ml of distilled water and was heated up to 350 K within half an hour. The resulting solution was kept at this temperature for a range between 3 to 20 h which resulted in hydrolysis of the alkoxide and formation of boehmite precipitation. The solutions were then mixed with a quantity of nitric acid with H+/alkoxide molar ratio in the range of 0.08–0.2 as the peptizing agent and were refluxed for 20 h. This resulted in formation of boehmite sols with different mean particle sizes which were characterized by Dynamic Light Scattering (DLS) analysis and were used in a successive sol–gel coating process on a tubular α-alumina support (O.D = 13 mm, I.D = 10 mm, GMITM Corporation) to obtain a modified substrate. The substrate was then used in a dual element CVD process with tetra ethyl ortho silicate (TEOS) and aluminum tri-sec butoxide (ATSB) as precursors.27 A series of membranes with different thicknesses and compositions of top selective layer were prepared by carefully controlling of CVD time and amount of precursors.28Three membranes with different thickness, composition and therefore separation properties (labeled as M01 to M03) were used in the present investigation as membrane reactors for simultaneous production and purification of hydrogen via MSR reaction. Permeation tests were first carried out at 773–1073 K for each membrane by introducing an equimolar mixture of gases containing H2, CH4, CO, and CO2 in order to evaluate hydrogen separation factor. The permeation module for this test consisted of two concentric quartz tubes which membranes were placed and fixed at the middle of the inner tube. The module was then placed in a tubular furnace, as shown in Fig. 1, and heated up to the specified temperature with a rate of 2 K min−1. Nitrogen stream was used as the sweep gas, exerted on the outer side and permeated flow was analyzed using gas chromatograph (GC) analyzer (Agilent, 7890A).
Steam reforming of methane was conducted at a temperature range of 773–1073 K and at various pressures (1–10 bar) in the prepared nano-composite membrane reactor. The experimental set-up shown in Fig. 2 was specifically designed for MSR reaction to operate at high temperature and pressure. It comprised two concentric tubular sections which consisted of a stainless steel outer shell and the membrane tube. The outer shell was tightly screwed at both ends and carbon O-rings were used to fix the membranes inside the shell.
Ni/Al2O3 catalyst was prepared and used in this study. The catalyst was synthesized using a general wet impregnation method24 by loading 10 wt% of nickel on γ-alumina support. Briefly, 10.2 g of Ni(NO3)2·6H2O (Merck, 99.9%) was dissolved in 200 ml of distilled water. γ-Alumina powder (Merck, 99.5%) of 18 g was added to this solution and the mixture was stirred for 10–15 min. The water was then removed using the rotary evaporator and the final catalyst was obtained by drying the collected powder at 110 °C overnight followed by calcination at 1073 K for 6 h. BET analysis (Micrometrics, ASAP 2010) was conducted to estimate catalytic surface area, total pore volume and average pore size of the catalyst. Phase structure of the catalyst was characterized by X-ray diffraction (XRD) analysis (Philips-XL10). Temperature programmed reduction (TPR) was carried out to determine the number of reducible species present on the catalyst surface and the temperature at which the reduction of each species occurs to find the most efficient reduction conditions. The catalyst was crushed and sieved to sizes of 0.1–0.3 mm. A quantity of 4 g of this catalyst was used to make up a catalytic bed of 5 centimeters length to fill the membrane zone. This catalyst was reduced in hydrogen at 923 K for 3 h before MSR reaction tests.
The catalyst was loaded inside the inner side of the membrane tube, where permselective composite layer has been deposited. The reaction zone was supported by a 1 cm bed of inert quartz chips at both ends to provide better heating and mixing of reactants. The reactor was installed in an electric furnace and temperature was increased at a ramping rate of 1 K min−1 to the reaction temperature with respective counter-current nitrogen flows of 50 ml (NTP) per min and 100 ml (NTP) per min through the shell and tube side before introduction of hydrogen at a flow rate of 50 ml (NTP) per min for 3 h on the reaction zone (tube side) to reduce the catalyst.
After reduction, the reactants (methane and steam with a mixing ratio of S/C = 3) were continuously fed to the tube side of the membrane reactor while the counter-current nitrogen flow was continued as sweep gas through the shell side. Methane flow rate was set at 5 ml (NPT) per min for atmospheric pressure. As the ratio of steam to methane was kept constant, the overall inlet flow rate of reactants was increased proportionally with increasing pressure to keep the space velocity constant for a specific length of reactor. Table 1 presents volumetric flow rates of reactants used for membrane reactors in MSR reaction. Back pressure regulators were used to adjust the pressures on both sides of the reactor. Both of the streams were passed through a condenser to remove probable moisture before injection into an online gas chromatograph (Agilent7890A) to determine the compositions of the streams (H2, CH4, CO, CO2). Fig. 2 shows the schematic set-up for steam reforming reaction.
| P (bar G) | CH4 flow rate (ml min−1) | Steam flow rate (ml min−1) |
|---|---|---|
| 1 | 5 | 15 |
| 2 | 10 | 30 |
| 4 | 20 | 60 |
| 6 | 30 | 90 |
| 8 | 40 | 120 |
| 10 | 50 | 150 |
Methane conversion and hydrogen yield were calculated according to the following equations:
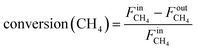 | (4) |
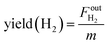 | (5) |
 is the inlet flow rate (mol s−1) of methane,
is the inlet flow rate (mol s−1) of methane,  is the outlet flow rate (mol s−1) of unreacted methane measured at the end of the reactor,
is the outlet flow rate (mol s−1) of unreacted methane measured at the end of the reactor,  is the outlet flow rate (mol s−1) of the produced hydrogen measured at the reactor outlet and m is weight of the catalyst in the reactor zone.
is the outlet flow rate (mol s−1) of the produced hydrogen measured at the reactor outlet and m is weight of the catalyst in the reactor zone.
3. Results and discussion
3.1. Membrane characterization
As discussed before, this type of membrane has been synthesized and characterized previously in detail by our team.28 In this research, we have used nitrogen sorption method (Micrometrics, ASAP 2010) to evaluate surface area, total pore volume, average pore diameter and porosity of the multi-layer membrane by analyzing the macroporous α-alumina support and mesoporous γ-alumina intermediate layer. The results (Table 2) show a decreasing trend in membrane characteristics due to decrease in porosity by adding successive layers which is in agreement with graded structure of the substrate. Scanning electron microscopy (SEM/FESEM) and Energy Dispersive Spectroscopy (EDS) were used to measure thickness of the selective layer for three membranes in order to evaluate Si effect on permeation properties. Simple SEM was used to evaluate composite layer for membranes with higher deposition time. However, for short deposition time the thickness is lower than 100 nm and therefore field emission images would give better results. Table 3 compares synthesis condition for membranes M01 to M03. A constant flow rate of silica carrier was used for coating the membranes. However, variation in deposition time results in different Si wt% and also different thickness of selective layer. Fig. 3 shows a cross-sectional SEM/FESEM images of M01 and M02 membranes. Macro-porous alumina support, intermediate γ-alumina layer and dense composite selective layer are clear in both images.| Sub-layer | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) | Average pore size (Å) | Porosity (%) |
|---|---|---|---|---|
| α-Alumina support | 144 | 0.45 | 1120 | 45–50 |
| γ-Alumina intermediate layer | 126 | 0.3 | 370 | 15–20 |
| Label | Deposition time (h) | Si wt% | Thickness of permselective layer |
|---|---|---|---|
| M01 | 3 | 6.08 | 75–80 nm |
| M02 | 4.5 | 7.93 | 80–90 nm |
| M03 | 6 | 8.24 | 90–110 nm |
 | ||
| Fig. 3 Cross-sectional image of nano-composite multi-layer membranes: (a) SEM image of M02 membrane with 4.5 h of deposition, (b) FESEM image of M01 membrane with 3 h of deposition. | ||
3.2. Catalyst properties
BET surface area of the Ni/Al2O3 catalyst was measured to be 120 m2 g−1. This area is comparable with previous researches, which were reported to be in the range of 110–150 m2 g−1.23–30 Total pore volume and average pore size of the catalyst were also measured to be 0.25 cm3 g−1 and 82 Å, respectively.X-ray diffraction (XRD) analysis was used to characterize the crystal structure of the prepared membrane and the alumina support phase (alpha or gamma), and also to ensure that no promoter is remained in the catalyst structure. As indicated in Fig. 4, the first pattern is the γ-alumina support with mesoporous structure, while the second one represents the Ni/Al catalyst after 3 h of calcination at 823 K. The resulting patterns show standard peaks for nickel and γ-alumina in the catalyst with good dispersion of nickel on alumina substrate. According to the diffraction patterns, alumina substrate is amorphous, while Ni shows a cubic crystal structure.
 | ||
| Fig. 4 XRD patterns of: (a) alumina substrate, (b) Ni/Al2O3 catalyst after 3 h of calcination at 823 K. | ||
TPR analysis result for catalyst after calcination at 823 K is demonstrated in Fig. 5. The figure shows two main peaks for catalyst reduction at about 753 K and 1143 K. The first peak with a hydrogen consumption of about 900 a.u. is directly related to reduction of Ni2+ to NiO and indicates a weak bond between metal and substrate; while the second peak with hydrogen consumption of less than 400 a.u. is related to reduction to NiAl2O4 in which nickel particles make strong bonds with substrate and reduction occurs at higher temperature.
3.3. Permeation properties of membranes
Effect of temperature on permeation properties of membranes for an equimolar mixture of hydrogen, carbon monoxide, carbon dioxide and methane was investigated for three membranes, M01, M02 and M03. A trans-membrane differential pressure of 1 bar was exerted as the driving force for gas permeation. Separation factor (SF) was defined as the permeance ratio of H2 to other gases through membrane. The results are shown in Fig. 6 with an error bar of 2%. These results demonstrate that although permeation flux predictably decreases with decreasing temperature, it still remains at an acceptable range of 5–6 × 10−7 mol m−2 s−1 Pa−1 for these membranes. These permeation values are considerably higher than reported permeation through silica membranes.29 The results also indicate that gas permeation through the membrane bulk in the gas mixture does not vary significantly comparing to single gas tests, as the hydrogen separation factor obtained here is almost the same as ideal selectivity which was achieved previously.28 This suggests that gas molecules of different species have no major interaction with one another or with membrane walls. Moreover, although permeance decreases with increasing CVD time and therefore thickness of the selective layer, all permeation curves obey the same trend which means that they have the same permeation mechanism. | ||
| Fig. 6 Gas permeation and separation factor of three membranes with different CVD time, as a function of temperature at 1 bar pressure. | ||
3.4. Effect of temperature on performance of the membrane reactor
The performance of membrane reactor is demonstrated mainly showing that the CH4 conversion and H2 yields could overcome the equilibrium values, in which the level of enhancement depended on H2 permeance of the membrane. In this section, effect of temperature on methane conversion at 1 bar pressure in the membrane reactor is shown in Fig. 7. For all membranes effect of hydrogen removal from the reaction zone is clearly observable as the methane conversions increase to higher values at all temperatures comparing to equilibrium conversion. This result is well in agreement with Le Chatelier's principle which claims that the system readjusts itself to counteract the effect of the applied change (concentration) and a new equilibrium is established.15 It is also interesting to note that despite better selectivity of membranes with longer CVD time, they show lower conversion of methane in membrane reactor (MR) as they have lower permeance. This demonstrates that permeation flux has a dominant effect on the conversion of reactants in MSR reaction.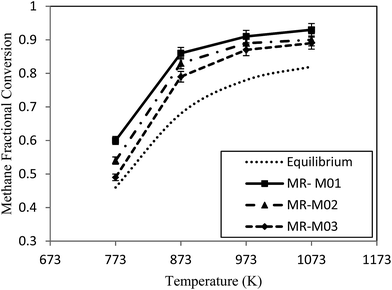 | ||
| Fig. 7 Effect of temperature on methane conversion at 1 bar for three membranes (M01, M02, M03) in the membrane reactor. | ||
H2 yield in the membrane reactor is presented in Fig. 8. This yield is actually the sum of yields in the permeate and the retentate sides which reflects the amount of produced hydrogen through the reaction. For all membranes in the membrane reactor, H2 yields slightly increased with increasing temperature which reveals that the reaction is clearly endothermic. On the other hand, H2 yields for different membranes followed the same trend as CH4 conversion and decreased with increasing CVD time. This is due to the fact that hydrogen yield is directly related to the conversion of reactants so that more conversions result in higher amounts of products in reaction zone.
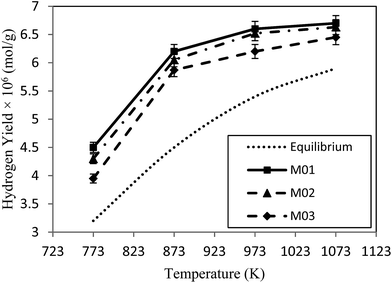 | ||
| Fig. 8 Hydrogen yield as a function of temperature at 1 bar for three membranes (M01, M02, M03) in the membrane reactor. | ||
3.5. Effect of pressure on performance of the membrane reactor
Effect of pressure on the MSR reaction in a range of 1–10 bar trans-membrane differential pressure was investigated at 773 K. This temperature was chosen because one of the most important aims of using MR is lowering reaction temperature. The equilibrium methane conversions and the experimental conversions in the MR for three membranes were plotted in Fig. 9. It is shown that all curves have a similar decreasing trend with increasing pressure as the MSR reaction is not thermodynamically favored at high pressures due to the net increase of moles on the product side.30 Enhancing effect of separation through the membrane on the CH4 conversion is clearly observed with higher conversion values at all pressures in membrane reactors. However, as the H2 permeation is not high enough, it cannot overcome the effect of increase in moles in the reaction zone, and therefore methane conversion decreases. | ||
| Fig. 9 CH4 conversion vs. pressure at constant temperature of 773 K for three membranes (M01, M02, M03) in the membrane reactor. | ||
Yield of hydrogen as a function of pressure is shown in Fig. 10. The results show an increasing trend with pressure for H2 yield and much higher yields are obtained in the MR than equilibrium condition. This might be somehow controversial as hydrogen yield normally has a direct relation with methane conversion, but here it follows an opposite trend. This is because of basics of membrane separation which is intensified by increasing pressure as the driving force for gas permeation. Therefore, by increasing pressure in MR, permeation, and not necessarily methane conversion, would increase which is favorable due to extracting more products from reaction zone. Membrane thickness has the same effect as before, so that membranes with higher CVD time have lower hydrogen yield because of lower permeation through the walls.
 | ||
| Fig. 10 H2 yield as a function of pressure at constant temperature of 773 K for three membranes (M01, M02, M03) in the membrane reactor. | ||
4. Conclusions
Ceramic membranes with a graded structure and different thicknesses of the composite layer were used as membrane reactors for methane steam reforming reaction. Permeation properties of these membranes were tested in an equimolar mixture at various temperatures and the results showed good permeation and acceptable separation of hydrogen. Effect of temperature and pressure on reactor performance of the membrane reactors showed higher methane conversion and hydrogen yield compared to the equilibrium condition even at lower temperatures. This suggests these membranes as a reliable substitution for conventional packed bed reactors. These results also indicate that although membranes with higher thickness of selective layer have higher selectivity for H2, they suffer from lower permeation flux, which in comparison is a more important factor of performance for membrane reactors.Acknowledgements
The authors are thankful of Research Institute of Petroleum Industry (RIPI) to provide all the required equipment and laboratory for this research. We would also like to thank Iran national Science Foundation (INSF) as the financial supporter of the present investigation.References
- S. Araki, N. Mohri, Y. Yoshimitsu and Y. Miyake, Synthesis, characterization and gas permeation properties of a silica membrane prepared by high-pressure chemical vapor deposition, J. Membr. Sci., 2007, 290, 138–145 CrossRef CAS.
- T. Tsuru, H. Shintani, T. Yoshioka and M. Asaeda, A bimodal catalytic membrane having a hydrogen-permselective silica layer on a bimodal catalytic support: Preparation and application to the steam reforming of methane, Appl. Catal., A, 2006, 302, 78–85 CrossRef CAS.
- J. Tong and Y. Matsumura, Effect of catalytic activity on methane steam reforming in hydrogen-permeable membrane reactor, Appl. Catal., A, 2005, 286, 226–231 CrossRef CAS.
- R. W. Spillman, Economics of gas separation membranes, Chem. Eng. Prog., 1989, 85, 41–62 CAS.
- S. Adhikari and S. Fernando, Hydrogen membrane separation techniques, Ind. Eng. Chem. Res., 2006, 45, 875–881 CrossRef CAS.
- G. Q. Lu, J. C. Diniz da Costa, M. Duke, S. Giessler, R. Socolow, R. H. Williams and T. Kreutz, Inorganic membranes for hydrogen production and purification: A critical review and perspective, J. Colloid Interface Sci., 2007, 314, 589–603 CrossRef CAS PubMed.
- G. Barbieri, G. Marigliano, G. Perri and E. Drioli, Conversion temperature diagram for a palladium membrane reactor. Analysis of an endothermic reaction: Methane steam reforming, Ind. Eng. Chem. Res., 2001, 40, 2017–2026 CrossRef CAS.
- M. W. Lee, Separation Membrane Development for Hydrogen, Westinghouse Savannah River Company, Siken, SC, 2000 Search PubMed.
- D. Edlund, Hydrogen Separation and Purification using Dense Metallic Membranes, DOE Hydrogen Separation and Purification Technologies, Arlington, VA, 2004 Search PubMed.
- S. Uemiya, N. Sato, H. Ando, T. Matsuda and E. Kikuchi, Steam reforming of methane in a hydrogen-permeable membrane reactor, Appl. Catal., 1991, 67, 223–230 CrossRef CAS.
- J. Shu, B. P. A. Grandjean and S. Kaliaguine, Methane steam reforming in asymmetric Pd- and Pd-Ag/porous SS membrane reactors, Appl. Catal., A, 1994, 119, 305–325 CrossRef CAS.
- A. Iulianelli, G. Manzolini, M. De Falco, S. Campanari, T. Longo, S. Liguori and A. Basile, H2 production by low pressure methane steam reforming in a Pd-Ag membrane reactor over a Ni-based catalyst: Experimental and modeling, Int. J. Hydrogen Energy, 2010, 35, 11514–11524 CrossRef CAS.
- S. Kitao, H. Kameda and M. Asaeda, Gas separation by thin porous silica membrane of ultra-fine pores at high temperature, Membrane, 1990, 25, 222–228 CrossRef.
- R. S. A. de Lange, J. H. A. Hekkink, K. Keizer and A. J. Burggraaf, Formation and characterization of supported microporous ceramic membranes prepared by sol–gel modification techniques, J. Membr. Sci., 1995, 99, 57–75 CrossRef CAS.
- R. S. A. de Lange, K. Keizer and A. J. Burggraaf, Analysis and theory of gas transport in microporous sol–gel derived ceramic membranes, J. Membr. Sci., 1995, 104, 81–100 CrossRef CAS.
- A. K. Prabhu, A. Liu, L. G. Lovell and S. T. Oyama, Modeling of the Methane Reforming Reaction in Hydrogen Selective Membrane Reactors, J. Membr. Sci., 2000, 177, 83–95 CrossRef CAS.
- B. N. Nair, T. Okubo and S.-I. Nakao, Structure and separation properties of silica membranes, Membrane, 2000, 25, 73–81 CrossRef CAS.
- G. R. Gavalas, C. E. Megiris and S. W. Nam, Deposition of H2-permselective SiO2 films, Chem. Eng. Sci., 1989, 44, 1829–1835 CrossRef CAS.
- T. Okubo and H. Inoue, Introduction of specific gas selectivity to porous glass membranes by treatment with tetra ethoxy silane, J. Membr. Sci., 1989, 42, 109–115 CrossRef CAS.
- A. K. Prabhu and S. T. Oyama, Hydrogen selective ceramic membranes: application to the transformation of greenhouse gases, J. Membr. Sci., 2000, 176, 233–248 CrossRef CAS.
- M. Nomura, T. Yamaguchi, I. Kumakiri and S. I. Nakao, Permeation properties and microstructures of porous inorganic membranes, Membrane, 2001, 23, 124–133 CrossRef.
- M. Nomura, K. Ono, S. Gopalakrishnan, T. Sugawara and S. I. Nakao, Preparation of a stable silica membrane by a counter diffusion chemical vapor deposition method, J. Membr. Sci., 2005, 251, 151–160 CrossRef CAS.
- B. N. Lukyanov, D. V. Andreev and V. N. Parmon, Catalytic reactors with hydrogen membrane separation, Chem. Eng. J., 2009, 154, 258–266 CrossRef CAS.
- J. Tong, Y. Matsumura, H. Suda and K. Haraya, Experimental study of steam reforming of methane in a thin Pd-based membrane reactor, Ind. Eng. Chem. Res., 2005, 44, 1454–1459 CrossRef CAS.
- R. V. Siriwardane, J. A. Poston Jr, T. H. Lee, S. E. Dorris and U. Balachandran, Characterization of ceramic hydrogen separation membrane with varying nickel concentrations, Appl. Surf. Sci., 2000, 167, 34–40 CrossRef CAS.
- E. Kikuchi, Membrane reactor application to hydrogen production, Catal. Today, 2000, 56, 97–105 CrossRef CAS.
- M. Amanipour, E. Ganji Babakhani, A. Safekordi, A. Zamaniyan and M. Heidari, Effect of CVD parameters on hydrogen permeation properties in a nano-composite SiO2–Al2O3 membrane, J. Membr. Sci., 2012, 423–424, 530–535 CrossRef CAS.
- M. Amanipour, A. Safekordi, E. Ganji Babakhani, A. Zamaniyan and M. Heidari, Effect of synthesis conditions on performance of a hydrogen selective nano-composite ceramic membrane, Int. J. Hydrogen Energy, 2012, 37, 15359–15366 CrossRef CAS.
- S. J. Khatib and S. T. Oyama, Silica membranes for hydrogen separation prepared by chemical vapor deposition (CVD), Sep. Purif. Technol., 2013, 111, 20–42 CrossRef CAS.
- D. Lee, L. Zhang, S. T. Oyama, S. Niu and R. F. Saraf, Synthesis, Characterization and Gas Permeation Properties of a Hydrogen Permeable Silica Membrane Supported on Porous Alumina, J. Membr. Sci., 2004, 231, 117–128 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



