Synthesis and characterization of LiNi0.48Co0.18Mn0.3Mg0.02Ti0.02O2 as a cathode material for lithium ion batteries
Abstract
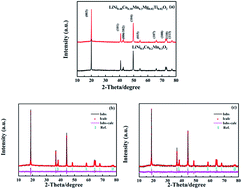
The layered oxide material LiNi0.48Co0.18Mn0.3Mg0.02Ti0.02O2 has been synthesized via a co-precipitation assisted solid-phase method, and its crystal structure, morphology and electrochemical properties have been systematically investigated. Rietveld refinement of its X-ray diffraction data indicates a higher degree of the well-ordered crystallographic form, which provides LiNi0.48Co0.18Mn0.3Mg0.02Ti0.02O2 with superior cycle performance and rate capability. The initial discharge capacities of the electrode are 151.5 mA h g−1, 140.1 mA h g−1, 137.1 mA h g−1, 125.2 mA h g−1 and 115.3 mA h g−1 at the current of 0.5C, 1C, 2C, 3C and 5C, respectively. After 100 cycles at the same rates, 94%, 96%, 96%, 94% and 93% of the initial discharge capacity are retained. The improved electrochemical properties are attributed to the decrease in particle size and suppression of cation mixing due to doping with Mg and Ti. The results of this work indicate that LiNi0.48Co0.18Mn0.3Mg0.02Ti0.02O2 is a promising cathode material for Li-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...