Controllable wetting state high adhesion hydrophobic surface†
Zeqing Wang,
Yuehua Cong and
Baoyan Zhang*
Northeastern University, P. O. Box 332, Wenhua Road, Lane 3 No. 11, Heping District, 110004, Shenyang, Liaoning Province, China. E-mail: byzhang2005@126.com
First published on 18th July 2016
Abstract
High adhesion hydrophobic surfaces with high apparent contact angles were achieved by a convenient approach. The morphology of the high adhesion surface both at the micrometre scale and at the nanometre scale can be changed by variation in the amount of carboxyl functionalized multi-wall carbon nanotubes. The contact angle values for the as-prepared surfaces are different, increasing from about 122.8° to 151.4° by increasing the amount of MWCNT–COOH. The as-prepared surface with a tunable adhesion can be achieved and the maximum volume of the water droplet adhered on the surface can be adjusted from about 5 μL to 35 μL. The variation in the contact angle and the maximum volume of the water droplet adhered on the surface are both attributed to the different morphologies of the surfaces. To analyze the correlation between the surface morphology and water droplet wetting, we suggest that the wetting state of the water droplet on the surface is changed, which results from the appearance of the micro/nano scale structure. Furthermore, a process of water droplet no-loss transport was achieved by the difference in adhesion of the surface.
Introduction
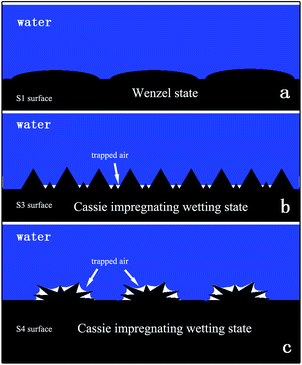
In nature, different wetting phenomena are observed everywhere, for example, the leaves of many plants or insect legs exhibit a unique self-cleaning property. Water droplets can easy roll off these surfaces. This phenomenon has attracted the attention and interest of researchers in many fields. Different wetting states were discovered on those surfaces. In general, Wenzel's state and Cassie's state are the two common states.1–3 In Wenzel's state, water droplets pin onto the surface at a wettable mode that results in a high contact angle (CA) hysteresis (high slide angle). In contrast, water droplets in Cassie's state adopt a non-wettable mode. In that case, air is trapped between the surface and the water droplet, and thus the three-phase contact line is discontinuous. The water droplet is suspended by the trapped air on a surface. Thus, the hysteresis in Cassie's state is lower than in Wenzel's state and the water droplets can easily roll down the surface. Nowadays, more and more wetting states have been found, i.e., the Lotus state, a special Cassie state, which can be observed on lotus leaf surfaces, and a superhydrophobic state with a high adhesion such as the Gecko state4 and the Cassie impregnating wetting state.In recently years, more and more researchers have focused on controlling the wetting states due to their potential applications in many fields5–17 such as microdroplet transportation, self-cleaning and anti-ice. In these fields, the adhesive property of a surface plays an important role. Thus, a new strategy to control the adhesion of the surface has received more attention by many researchers at present.16,18–24 There are two important factors to affect the adhesive property of the surface. One is the top structure, and the other is the chemical composition. Nowadays, much research has been reported on these surfaces such as duplicated petal structures using poly(vinyl alcohol) or polystyrene,4 fabricated periodic microstructures on a metal surface via laser irradiation,20 and a modified surface by a chemical method.21
At present, carbon nanotubes have been used to make many unique materials with major applications in numerous areas. Carbon nanotubes have also been used in the preparation of hydrophobic materials. A superhydrophobicity carbon nanotube-based film was produced by acetone-treatment.25 The acetone-treated carbon nanotubes exhibit superhydrophobicity behaviour and an ultralow CA hysteresis. Thus, a water droplet would easily bounce off the surface. To enhance the adhesion between water and the surface, Bhalchandra A. Kakade designed an ingenious method for changing the water wetting state on multi-wall carbon nanotubes by varying the electric field.26 However, all these methods are more or less limited by the experimental conditions, the price of the material and by the complexity of the method. Thus, fabricating a controllable, high adhesion hydrophobic surface is still a challenge.
In this study, high adhesion hydrophobic surfaces with a high apparent CA were achieved by a simple process. The morphology of the surface was tuned by changing the amount of carboxyl functionalized multi-wall carbon nanotubes (MWCNT–COOH). By controlling the amount of MWCNT–COOH, the CA values and the adhesion (reflected by the maximum volume of the water droplet adhered on the surface) were tuneable. Such changes are ascribed to the variation in the morphology of the sample surface, which may result in a different wetting state for the water on the surface. Furthermore, we demonstrated a process of water droplet no-loss transport with a variation of adhesion.
Experiment
Materials
MWCNT–COOH, which were synthesized by oxidizing MWCNT in a mixture of sulfuric acid and nitric acid, were purchased from the Chengdu Organic Chemicals Co. Ltd. Parameters for the MWCNT–COOH are as follows: the purity is 95 wt%, the outer diameter is less than 8 nm, the inner diameter is in the range of 2–5 nm, the length is about 10–30 μm, and the special surface area is more than 500 m2 g−1. The 1-octadecanol was purchased from the Sinopharm Chemical Reagent Co. Ltd.Method
The MWCNT–COOH powder was dispersed in chloroform, and then a 10 wt% 1-octadecanol/chloroform solution was added into the dispersion under strong mechanical stirring for 1 h. A homogeneous dispersion was obtained. With the variation of additive amount of MWCNT–COOH powder (as illustrated in Table 1), the samples S1, S2, S3 and S4 were fabricated.| Sample | MWCNT–COOH/mg | Chloroform/μL | 10 wt% 1-octadecanol/chloroform solution/μL |
|---|---|---|---|
| S1 | 13.72 | 500 | 150 |
| S2 | 25.90 | 500 | 150 |
| S3 | 37.97 | 500 | 150 |
| S4 | 46.72 | 500 | 150 |
50 μL of the as-prepared dispersion was air-brushed onto the surface of a glass wafer. The chloroform was slowly removed under ordinary pressure at room temperature and dried in a vacuum at room temperature for 8 h. The high adhesion surface was fabricated onto the glass wafer.
Characterization
The Fourier transform infrared (FTIR) spectra were obtained using a PerkinElmer Spectrum spotlight 300, which can collect surface functional group information. The samples were scanned five times at a resolution of 4 cm−1. The reported spectra are an average of five scans.The CA of the as-prepared surface was characterized by a water contact angle meter (JC2000D1, Shanghai Zhongchen digital technology apparatus Co., Ltd) with a 5 μL water droplet at ambient temperature. For each reported value, the CA was measured at five different positions on the surface.
The method to measure the maximum volume of the water droplets adhered on the high adhesion surface is as follows: first, the water droplet (5 μL) was dropped on the surface; then the surface was overturned 180° slowly, with the water droplet still adhered to the surface. 5 μL water droplets were added into the water droplet adhered on the surface, one at a time, until the overturned water droplet drop down from the surface. The maximum volume of the water droplets adhered on the surface was obtained by measuring at three different positions of the same sample surface by this method, and results were obtained by averaging.
The feature information of the sample surface was observed by using a field emission scanning electron microscope (FESEM) (Carl Zeiss Ultra Plus).
The adhesion phenomenon of the water droplet on the high adhesion surface was captured by a digital camera (canon 6d lens with sigma 35 mm F 1.4 DG). A water droplet (5 μL) was first adhered on a needle and then the needle was moved down at a constant speed of 2 μm s−1 until the water droplet made contact with the surface, at which point the needle was removed, and the process was captured by a digital camera.
The process of water droplet no-loss transport was captured by a digital camera (canon 6d lens with sigma 35 mm F 1.4 DG).
Result and discussion
High adhesion surface characterization
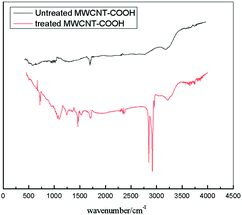
A typical FTIR spectrum for the untreated MWCNT–COOH is shown in Fig. 1 (black line). As shown in Fig. 1, an obvious peak around 1740 cm−1 can be observed, which is normally assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration in the carboxyl group, can be found in the spectrum of the black line. A typical FTIR spectrum of the treated MWCNT–COOH is shown in Fig. 1 (red line). Three new peaks appear at wavenumber of around 2930 cm−1, 2855 cm−1 and 3300 cm−1 can be found from the red line in Fig. 1. Of the three peaks, the peaks at wavenumber of around 2930 cm−1 and 2855 cm−1 are normally assigned to the C–H stretching vibration in the alkyl group, which means that some alkyl groups were introduced into the treated MWCNT–COOH by treating the MWCNT–COOHs with 1-octadecanol; and the peak at a wavenumber of around 3300 cm−1 is normally assigned to a hydroxyl group. From Fig. 1, we know that both the hydrophilic group (the carboxyl group and hydroxyl group) and the hydrophobic group (long alkyl chain) exist on the treated MWCNT–COOH surface.
O stretching vibration in the carboxyl group, can be found in the spectrum of the black line. A typical FTIR spectrum of the treated MWCNT–COOH is shown in Fig. 1 (red line). Three new peaks appear at wavenumber of around 2930 cm−1, 2855 cm−1 and 3300 cm−1 can be found from the red line in Fig. 1. Of the three peaks, the peaks at wavenumber of around 2930 cm−1 and 2855 cm−1 are normally assigned to the C–H stretching vibration in the alkyl group, which means that some alkyl groups were introduced into the treated MWCNT–COOH by treating the MWCNT–COOHs with 1-octadecanol; and the peak at a wavenumber of around 3300 cm−1 is normally assigned to a hydroxyl group. From Fig. 1, we know that both the hydrophilic group (the carboxyl group and hydroxyl group) and the hydrophobic group (long alkyl chain) exist on the treated MWCNT–COOH surface.
Features of high adhesion surface
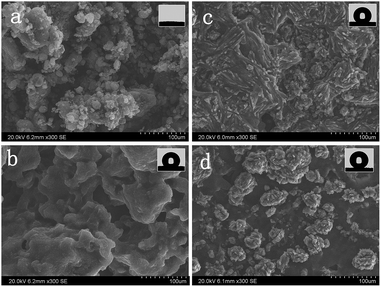
In general, the surface morphology essentially governs the wetting properties of a solid. Thus, the features of each sample were investigated. The morphology of the MWCNT–COOH without any treatment is shown in Fig. 2a, and the magnified SEM images are shown in Fig. S1 and S2 in the ESI.† As shown in Fig. 2, many ball-like features (the magnified image is shown in Fig. S2†) were found. A random network was observed clearly in Fig. S1.† The networks were formed on the glass wafer surface with plenty of empty interspaces between each MWCNT–COOH. A phenomenon in which many MWCNTs–COOH are assembled is shown in Fig. S2;† the phenomenon was attributed to van der Waals forces.27When 1-octadecanol was added, the surface morphology changed. The ball-like structures vanished and a flat structure appeared (Fig. 2b). It is worth noting that many remarkable groove-like structures (microscale and nanoscale) appeared on the flat structure surface with the increasing amount of MWCNT–COOH (Fig. 2c), and a more clearly detailed structure is shown in Fig. S3.† Further increasing the amount of MWCNT–COOH, it was found that the continuous structure started to break up. Instead, a cluster structure appeared, as shown in Fig. 2d. The size of the clusters ranged from 20 μm to 50 μm. When the cluster was amplified, more detailed information was found. The magnified SEM image in Fig. S4† clearly revealed that these clusters exhibited thorn structures on the nanometre scale with more nanostructures formed. From the abovementioned analysis, it is found that the morphology of the high adhesion surface both on the micrometre scale and on the nanometre scale can be changed by variation of the amount of MWCNT–COOH.
Wetting property of high adhesion surface
Insets in Fig. 2 are the shapes of the water droplet on the surface. The CA values of the as-prepared surfaces were measured by the contact angle meter. Before the treatment, the MWCNT–COOH surface demonstrated a relatively hydrophilic property (as shown in Fig. 3a). When a water droplet is dropped onto the surface of the MWCNT–COOH film, it immediately disappears and just leaves a trace of water droplet on the surface, whereas the high adhesion surface exhibits a superhydrophobic behavior with a high CA of about 151.4° (as shown in Fig. 3b). Because of the strong water repellence of the surface, the water droplet maintains a nearly spherical sharp.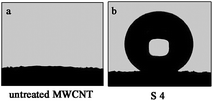 | ||
| Fig. 3 A image of the water droplet on the surface of the (a) untreated MWCNT surface and the (b) high adhesion surface. | ||
The change from hydrophilic to hydrophobic may be attributed to variation of the surface morphology and the hydrophilicity of the MWCNT–COOH. It is suggested that there are hydrogen-bond interactions between the MWCNT–COOH and the 1-octadecanol on the MWCNT–COOH surface (as shown in the red frame of Fig. 4) while treating the MWCNT–COOH with 1-octadecanol. Moreover, the 1-octadecanol linked onto the MWCNT–COOH can attract more 1-octadecanol around the MWCNT–COOH by van der waals forces. In general, the carboxyl groups are located on the end and the wall of the MWCNT–COOH, which were both treated by an acid-mixture.28 Consequence, the MWCNT–COOH surface was coated by 1-octadecanol, and the network structure between the MWCNT–COOH was filled by 1-octadecanol. Thus, the treated MWCNT–COOH surface has more hydrophobic groups and less network structure, which results in the wetting characteristics changing from hydrophilic to hydrophobic.
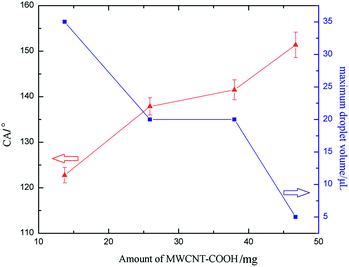
The CA values for each sample are S1: 122.8°, S2: 137.9°, S3: 141.5° and S4: 151.4°. It can be observed that the CA value for the as-prepared surface is different and increases from about 122.8° to 151.4°. It is found that by increasing the amount of MWCNT–COOH, the morphology changed, especially the increase in the roughness factor (microstructure appeared which can be clearly observed in Fig. 2b and c), which results in the change of the CA value for the water droplet on the high adhesion surface (as shown in Fig. 6).
Adhesive property of high adhesion surface
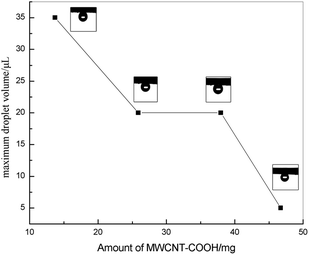
In Cassie's state, the water droplets adopt a non-wetting mode. Many air pockets may be trapped between the solid voids under the water droplet. Because the air pockets were trapped on the surface, a water droplet can be suspended on the solid surface. Thus, the adhesive force of the water droplet, which contacts the solid surface, is low. The water droplet can roll off easily. In contrast, in Wenzel's state, the water droplet resides in the wetting mode and pins to the surface. As a result, a high adhesive force would be observed. From the abovementioned analysis, the water droplet would reside in the Wenzel state on the high adhesion surface, which is caused by the high adhesive phenomenon.The high adhesion surface shows a high CA hysteresis, in which the water droplet cannot roll off when the surface is titled vertically, or even reversed. The adhesive characteristic of the surface can be reflected by the volume of the droplet adhered on the surface. For a small water droplet, the weight of the droplet is smaller than the surface tension force, and thus the droplet will be adhered onto the surface. When the volume of the water droplet increases, the balance between the weight of droplet and the surface tension force is broken, and then the droplet adhered on the surface will fall.4 In this study, a high adhesion surface was fabricated. A water droplet on the high adhesion surface cannot roll off when the surface is turned upside down. The typical shape of the water droplet on the surface (S2) is shown in Fig. 5. The volume of the water droplet on the surface (S2) is 10 μL. The maximum volume of the water droplet adhered on the high adhesion surface is shown in Fig. 6 (blue line) and the shape of the water droplet on the reversed surface is shown in Fig. 7. As shown in Fig. 6 (blue line), it can be found that the maximum volume of the water droplet decreases as the amount of MWCNT–COOH is increased.
In addition, images of the adhesion phenomenon of the water droplet on the surface were captured. The typical images of the adhesion phenomenon on the high adhesion surface are exhibited in Fig. 8. As shown in the L row (S4 surface) of Fig. 8, first, a water droplet moved down and contacted the surface (shown from a to c in Fig. 8 L row). Then, the water droplet separated from the surface with the needle moving upward (shown in d in L row of Fig. 8), and in this process, the shape of the water droplet remained spherical and none of the water droplet adhered to the surface.
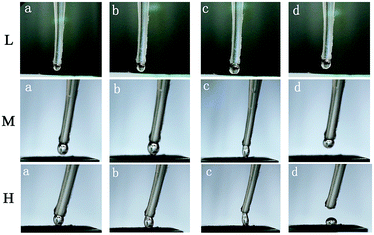 | ||
| Fig. 8 The adhesion phenomenon of water droplet on the high adhesion surface. The surface in L row is S4, the surface in M row is S3, and the surface in H row is S1. | ||
The same process was performed on the S3 surface. Results are shown in the M row in Fig. 8. As shown in the M row of Fig. 8, we find that the phenomenon is rather different. The water droplet was pulled from spherical to elliptical, while a distance between the surface and the water droplet was increased. The triple-phase contact lines were changed and the contact area was reduced until the water droplet broke away from the surface. Furthermore, the water droplet was transported from the needle to the surface (S1 surface) due to the high adhesion, as shown in the H row of Fig. 8. The high adhesion of the surface maybe related to the functional groups on the surface. From Fig. 1, we know that the carboxyl group and the hydroxyl group, which are hydrophilic functional groups, exist on the treated MWCNT–COOH surface. Thus, it is suggested that the hydroxyl and carboxyl groups of the surface may hold water molecules like a surfactant. The hydrophilic functional groups play the role of an anchor, which can hold the water droplet on the surface. Because the abovementioned phenomenon occurred on the surface, a high adhesion hydrophobic surface was obtained.
As described above, although the entire as-prepared surfaces have a similar adhesivity, the adhesion ability is rather different. Increasing the amount of MWCNT–COOH would decrease the adhesion of the surface. According to the relationship between the surface morphology and the amount of MWCNT–COOH, we believe that different adhesive properties for the high adhesion surfaces are mainly attributed to the surface morphology.
It is common that the surface adhesion is a product of the liquid and solid interfacial interactions and contact area (eqn (1)).29
| F = kIA | (1) |
On the S1 surface, the morphology of the surface is flat (as shown in Fig. 2b), a water droplet can wet the surface and the wetting state is the Wenzel state (Fig. 9a). In this state, the contact area is largest and the surface adhesion is bigger than for other sample surfaces. With the variation in the amount of MWCNT–COOH, the microstructure and nanostructure appeared on the surface S3 (as shown in Fig. 2c). The feature of the water droplet contact mode on surface S3 is that the water droplet can wet the microstructure, which is a result of the large scale of the microstructure, while the nanostructure remains dry. The droplet would reside in a Cassie impregnating wetting state (Fig. 9b). In this state, the nanostructure cannot be wet, which means many trapped air pockets were formed. The trapped air would decrease the water/solid surface contact area; consequently, the adhesion of the surface becomes low.
Further increasing the amount of MWCNT–COOH, a cluster structure appeared (as shown in Fig. 2d). The size of the cluster ranged from 20 μm to 50 μm; moreover, there are many thorns in the nanometre scale on the cluster surface (surface S4), as shown in Fig. S4.† On the surface, because the cluster structure is on the micrometre scale, the water droplet can wet the gaps between the clusters. However, the thorns are nanostructures, which cannot be wet by the water droplet on this surface, such that this surface not only has sufficient roughness for superhydrophobicity, but also has a high adhesive force.4 On the surface with such structure, the water droplet is in a Cassie impregnating wetting state (Fig. 9c). Air was sealed in the nanostructure by water and much trapped air was produced (as shown in Fig. 9c). The appearance of the trapped air is caused by a reduction in the contact area. Thus, the adhesion of surface S4 is lower.
As described above, it is found that by augmenting the amount of MWCNT–COOH, a change of the surface morphology, particularly the appearance of a micro-structure or micro/nano-structure, would result in the transition in the wetting state from the Wenzel state to the Cassie impregnating wetting state.
Water droplet no-loss transport
From the abovementioned analysis, these surfaces exhibit a high adhesion property. In addition, the adhesion force can be tuned by changing the amount of MWCNT–COOH. This ability allows for the high adhesion surface to be applied to a large range of applications. In this study, we demonstrated an application of these surfaces with no-loss transport. The process of water droplet no-loss transport is shown in Fig. 10. The S3 surface was formed on top of a plastic column. The S1 and S4 surfaces were formed on glass wafers. First, a water droplet (5 μL) was deposited on a low adhesion surface (S4 surface), as shown in Fig. 10a. Then, the S3 surface was brought into contact with the water droplet (Fig. 10b). When the S3 surface contacted the water droplet, the surface moved upward. The water droplet separated from the S4 surface and was transported to S3 surface due to the variation in adhesion. Subsequently, the water droplet adhered on the S3 surface came into close to S1 surface until contact with the S1 surface was made, and then the S3 surface moved upward. We observed that the shape of the water droplet was stretched. The water droplet was the transported to S1 surface, while the distance between the S1 surface and the S3 surface increased.Conclusion
In summary, the high adhesion hydrophobic surface, which has a tunable water adhesion and high CA, was obtained by treating MWCNT–COOH with 1-octadecanol. It is believed that the adhesion of the surface is ascribed to the water/solid contact area (A), which is controlled by the amount of MWCNT–COOH. The adhesion force is reflected by the maximum volume of water droplet adhered on the as-prepared surface. The maximum volume of the water droplet adhered on the high adhesion surface is nearly 30 μL. The phenomenon of tunable adhesive is attributed to the transition of the wetting state from a Wenzel state to a Cassie impregnating wetting state which results from the variation in the morphology (microstructure and nanostructure appeared) on the surfaces. Furthermore, we demonstrated an application for these surfaces with no-loss transport. It is hoped that these results and related methods may provide a new strategy for controllable water adhesion.Acknowledgements
The authors are grateful to the National Science Fundamental Committee of China, and HI-Tech Research and Development Program (863) of China. We also acknowledge the work done by Haibin Wang in taking the images of water droplets on the surfaces.Notes and references
- S. Wang and L. Jiang, Adv. Mater., 2007, 19, 3423–3424 CrossRef CAS.
- X. Yao, Y. L. Song and L. Jiang, Adv. Mater., 2011, 23, 719–734 CrossRef CAS PubMed.
- D. Hong, I. Ryu, H. Kwon, J.-J. Lee and S. Yim, Phys. Chem. Chem. Phys., 2013, 15, 11862–11867 RSC.
- L. Feng, Y. Zhang, J. Xi, Y. Zhu, N. Wang, F. Xia and L. Jiang, Langmuir, 2008, 24, 4114–4119 CrossRef CAS PubMed.
- J. Yang, H. Song, H. Tang, H. Ji and C. Li, Appl. Surf. Sci., 2013, 280, 940–944 CrossRef CAS.
- Z.-H. Yang, F.-C. Chien, C.-W. Kuo, D.-Y. Chueh and P. Chen, Nanoscale, 2013, 5, 1018–1025 RSC.
- Y. Zhao, M. Qin, A. Wang and D. Kim, Adv. Mater., 2013, 25, 4561–4565 CrossRef CAS PubMed.
- A. I. Aria and M. Gharib, Langmuir, 2014, 30, 6780–6790 CrossRef CAS PubMed.
- Z. Hu, X. Zhang, Z. Liu, K. Huo, P. K. Chu, J. Zhai and L. Jiang, Adv. Funct. Mater., 2014, 24, 6381–6388 CrossRef CAS.
- S. Moradi, P. Englezos and S. G. Hatzikiriakos, Langmuir, 2014, 30, 3274–3284 CrossRef CAS PubMed.
- M. T. Z. Myint, G. L. Hornyak and J. Dutta, J. Colloid Interface Sci., 2014, 415, 32–38 CrossRef CAS PubMed.
- X. Xu, G. Vereecke, C. Chen, G. Pourtois, S. Armini, N. Verellen, W.-K. Tsai, D.-W. Kim, E. Lee, C.-Y. Lin, P. Van Dorpe, H. Struyf, F. Holsteyns, V. Moshchalkov, J. Indekeu and S. De Gendt, ACS Nano, 2014, 8, 885–893 CrossRef CAS PubMed.
- H. Zhu, Z. Guo and W. Liu, Chem. Commun., 2014, 50, 3900–3913 RSC.
- F. De Nicola, P. Castrucci, M. Scarselli, F. Nanni, I. Cacciotti and M. De Crescenzi, Nanotechnology, 2015, 26, 145701 CrossRef PubMed.
- S. Feng, S. Wang, C. Liu, Y. Zheng and Y. Hou, Chem. Commun., 2015, 51, 6010–6013 RSC.
- H.-C. Huang and N. S. Zacharia, Langmuir, 2015, 31, 714–720 CrossRef CAS PubMed.
- J. M. Lee, S.-H. Lee and J. S. Ko, Sens. Actuators, B, 2015, 213, 360–367 CrossRef CAS.
- N. Wang, D. Xiong, Y. Deng, Y. Shi and K. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6260–6272 CAS.
- L. Tie, Z. Guo and W. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 10641–10649 CAS.
- J. Long, P. Fan, D. Gong, D. Jiang, H. Zhang, L. Li and M. Zhong, ACS Appl. Mater. Interfaces, 2015, 7, 9858–9865 CAS.
- H. Liu, Y. Ding, Z. Ao, Y. Zhou, S. Wang and L. Jiang, Small, 2015, 11, 1782–1786 CrossRef CAS PubMed.
- Z. Liu, Y. Feng and W. Li, RSC Adv., 2015, 5, 29017–29021 RSC.
- S. Park, J. Kim and C. H. Park, RSC Adv., 2016, 6, 45884–45893 RSC.
- R. Sahay, H. Y. Low, A. Baji, S. Foong and K. L. Wood, RSC Adv., 2015, 5, 50821–50832 RSC.
- L. Q. Zheng, Z. R. Li, S. Bourdo, K. R. Khedir, M. P. Asar, C. C. Ryerson and A. S. Biris, Langmuir, 2011, 27, 9936–9943 CrossRef CAS PubMed.
- B. A. Kakade, Nanoscale, 2013, 5, 7011–7016 RSC.
- N. B. Pramanik and N. K. Singha, RSC Adv., 2015, 5, 94321–94327 RSC.
- B. Kim and W. M. Sigmund, Langmuir, 2004, 20, 8239–8242 CrossRef CAS PubMed.
- Z. Cheng, M. Du, H. Lai, N. Zhang and K. Sun, Nanoscale, 2013, 5, 2776–2783 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14756a |
| This journal is © The Royal Society of Chemistry 2016 |