Fluorescence “turn on” detection of Cr3+ using N-doped-CDs and graphitic nanosheet hybrids†
Meiling Wanga and
Guowen Meng*ab
aKey Laboratory of Materials Physics, CAS Center for Excellence in Nanoscience, Anhui Key Laboratory of Nanomaterials and Nanotechnology, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei, 230031, China. E-mail: gwmeng@issp.ac.cn; Fax: +86-0551-65591434; Tel: +86-0551-65592749
bUniversity of Science and Technology of China, Hefei, 230026, China
First published on 26th July 2016
Abstract
Nitrogen-doped CDs and graphitic nanosheet hybrids (denoted as “N-doped-CDs@GNSs”) were firstly obtained by a simple, economical, and green preparative strategy using peanut shells. The N-doped-CDs@GNSs were used for fluorescence “turn on” detection of Cr3+ based on fluorescence resonance energy transfer, without further surface modification.
Carbon dots (CDs), as novel photoluminescent materials, have attracted much attention owing to their superior aqueous solubility, resistance to photobleaching, chemical inertness and low toxicity.1–7 As reported, CDs provide a wide variety of promising applications in fluorescence sensing and bio-imaging of Hg2+, Ag+, Cu2+, Fe3+, Zn2+, I− and superoxide anions.1 However, all of these sensors are based on CDs-fluorescence quenching (“turn off”) mechanism induced by the target ions, which suffers from multiple interferences caused by external quenchers or other environmental factors in practical applications. As for heavy metal ions, many kinds of different ions can quench the fluorescence of CDs through electron or energy transfer,1f thus leading to a very bad selectivity for specific heavy metal ions. In contrary, fluorescence enhancement (“turn on”) becomes a conceivable strategy to overcome the problems toward the selective detection of special heavy metal ions.1f It is also believed that “turn on” is preferable to “turn off” due to its higher target selectivity and less false positives.2 Thus two kinds of fluorescence sensors based on CDs-fluorescence enhancement for Hg2+ and Cr2O72− have been reported so far.1f,2 However, no fluorescence sensors for Cr3+ based on CDs-fluorescence enhancement have been reported, although two kinds of Cr3+ sensing methods using CDs-fluorescence quenching were publicated.8,9 And it should be noted that, both of the above mentioned CDs-based sensors show bad selectivity to Cr3+.8,9
Cr3+, as another chemical state of chromium, can bind to DNA and destroy cellular structure.10 Even low concentrations of Cr3+ in aqueous solutions can enter biological tissue, progressively enlarge along the food chain and finally harm human health. Thus Cr3+ analytical methods, with high sensitivity, selectivity and fast response, are exigent even for low concentrations of Cr3+. Up to now, several fluorescence-based probes for Cr3+ have been reported based on rhodamine derivatives and glutathione modified quantum dots.11–13 However, fabrications of those fluorescence sensing materials are complex, and all of them are based on organic fluorophores or semiconductor quantum dots consisting of toxic heavy metals (such as CdTe).13–15 Most importantly, all of the above mentioned Cr3+ sensors rely on fluorescence quenching. Therefore, if trace detection of Cr3+ in aqueous solutions can be realized by using inexpensive CDs-fluorescence enhancement, the results would be appealing.
Graphene is a famous superquencher of fluorophores due to the energy or electron transfer from excited fluorophores to the π state of graphene.16 Thus fluorescence resonance energy transfer (FRET) process may also take place between CDs and graphitic nanosheets in a similar way, with the former acting as the donor while the latter as the acceptor. Herein, we report for the first time the simple synthesis of a new kind of nitrogen-doped CDs (denoted as N-doped-CDs) and graphitic nanosheets (GNSs) hybrid (denoted as “N-doped-CDs@GNSs”) using low cost waste peanut shells and 1,6-hexamethylendiamine as raw materials, and their fluorescence “turn on” detection of Cr3+, as schematically shown in Fig. 1. This new kind of N-doped-CDs@GNSs hybrid is obtained by simply adding 2 ml 1,6-hexamethylendiamine into the initial hydrothermal raw materials (peanut shells) under strong alkali conditions, and used as obtained without further surface modification. In the strong alkali environment, part of the peanut shells was carbonized to N-doped-CDs, while the other part was stripped to pieces by the strong base and formed into GNSs with various groups on their surface (highlighted in blue on the surface of the GNSs in Fig. 1). It should be noted that there exists multivalent CH–π interaction between the π-conjugation of the GNSs and the CH– of 1,6-hexamethylendiamine on the surface of the N-doped-CDs.16 And this tight binding can bring N-doped-CDs and GNSs into appropriate proximity and induce FRET from the excited N-doped-CDs to the GNSs, being similar to the previous report.16 However, with the addition of Cr3+, the FRET process was partially inhibited by Cr3+ as a result of the competition between Cr3+ and the N-doped-CDs, and fluorescence enhancement was observed. Thus the N-doped-CDs@GNSs hybrid can be used for fluorescence “turn on” detection of Cr3+ in aqueous solutions. According to our experiments, [Cr3+] in the range of 0–700 nM can be linearly detected, and a detection lower limit of 0.7 nM has been achieved using this N-doped-CDs@GNSs hybrid. Furthermore, the practical application of the new N-doped-CDs@GNSs hybrid is proved by successfully fluorescence analysis of Cr3+ in real tap water and real lake water samples. It is exciting that no other common metal ions can interfere the fluorescent sensing of Cr3+ using this method. This is the first report of fluorescence sensors for Cr3+ based on CDs-fluorescence enhancement using the N-doped-CDs@GNSs hybrid. Therefore, our work opens a new avenue to the design of CDs-fluorescence sensor based on FRET.
 | ||
| Fig. 1 Schematic showing the fabrication of the N-doped-CDs@GNSs hybrid and its fluorescence sensing mechanism for Cr3+. | ||
The N-doped-CDs@GNSs hybrid was prepared by hydrothermal treatments of 1,6-hexamethylenediamine and the low cost carbon raw material, i.e., peanut shells, which compose a high quantity of carbon-rich lignin and cellulose. Typically, 1.2 g of the peanut shell, 2 ml 1,6-hexamethylenediamine and 40 ml 2 M NaOH solution were put into a 60 ml Teflon-lined autoclave and heated at 200 °C for 5 h. In the strong alkali environment, a part of the lignin and cellulose was hydrolyzed to small molecules and finally carbonized to N-doped-CDs. It should be noted that the surface of the N-doped-CDs was aminated by 1,6-hexamethylenediamine under the current experimental conditions, which is similar to our previous report.16 The other part of the peanut shells was stripped to pieces by the strong base and formed into GNSs under the high temperature and high pressure. The existed multivalent CH–π interaction between the π-conjugation of the GNSs and the CH– on the surface of the N-doped-CDs, brought the N-doped-CDs and the GNSs into close proximity and induced the formation of the N-doped-CDs@GNSs hybrid.15
The resultant products were collected by removing the large particles through centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 rpm for 15 min followed by filtering, and finally dried in a vacuum freeze drier for 48 h. The N-doped-CDs@GNSs hybrid was re-dispersed in DI-water at a concentration of 0.15 mg ml−1 for further characterization and use.
800 rpm for 15 min followed by filtering, and finally dried in a vacuum freeze drier for 48 h. The N-doped-CDs@GNSs hybrid was re-dispersed in DI-water at a concentration of 0.15 mg ml−1 for further characterization and use.
The detection of Cr3+ was performed at room temperature in DI-water (pH 7.0) solution. In a typical run, a given amount of Cr3+ solution was added to the 0.15 mg ml−1 N-doped-CDs@GNSs dispersion, and the fluorescence spectra were recorded after reaction for 1 min at room temperature. The sensitivity and selectivity measurements were conducted in triplicate.
Our experiments prove that the yield of the final product increases with the reaction temperature, reaction duration and the concentration of NaOH used. For the N-doped-CDs@GNSs hybrid obtained by using 1.2 g peanut shell and 2 M NaOH, reacted at 200 °C for 5 h, the yield reaches as high as 27%. Photos of the obtained N-doped-CDs@GNSs hybrid in solution and the dried N-doped-CDs@GNSs hybrid powder are shown in Fig. 2a and b, respectively. Typical TEM images with different magnifications (Fig. 2c and d) reveal that the product consists of N-doped-CDs (the round dark nanoparticles) uniformly distributed on the surface of the nanosheets (large-area shadow background). Crystalline structures with lattice spacing of about 0.24 and 0.32 nm were observed from the lattice-resolved TEM images taken from the nanosheets (lower-middle inset in Fig. 2d) and N-doped-CDs (lower-right inset in Fig. 2d) respectively, being consistent with the (002) and (1120) lattice planes of graphitic carbon, and this is similar to those reported previously.1i,6c,15 The surface elemental composition and chemical states of the resultant N-doped-CDs@GNSs hybrid were analyzed using the XPS technique (Fig. S1†), indicating that the N-doped-CDs@GNSs hybrid is mainly composed of C, O and N, and that multiple oxygen and nitrogen groups (NH2–) are formed on the surface of the N-doped-CDs@GNSs hybrid (details can be seen in Part S1 in the ESI†). Statistic counting of 70 N-doped-CDs shows that the diameters of the N-doped-CDs range from 1 to 5 nm with an average diameter of about 3.1 nm (see Fig. S2 in the ESI†), and a diameter distribution histogram is shown in Fig. 2e. The excitation-dependent-emission spectra of the N-doped-CDs@GNS hybrid are shown in Fig. 2f, with the excitation varying from 320 nm to 560 nm. The UV-vis spectrum of the N-doped-CDs@GNSs hybrid indicates that there exist two absorption peaks of 280 nm and 350 nm, respectively (Fig. 2g).
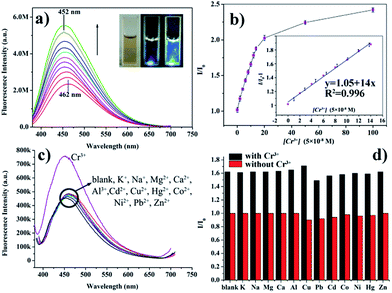
The feasibility of using such N-doped-CDs@GNSs hybrid for Cr3+ detection was explored. The N-doped-CDs@GNSs hybrid solution exhibits an emission peak at 462 nm with excitation of 360 nm (Fig. 3a). However, fluorescence quantum yield of just 0.1% was evaluated using rhodamine 6G as a reference. This low quantum yield might be attributed to the energy or electron transfer from the excited N-doped-CDs to the π state of GNSs.15 However, there exists an obvious increase of the fluorescence intensity (Fig. 3a) with the successive addition of Cr3+, indicating that Cr3+ can effectively enhance the fluorescence of the N-doped-CDs@GNSs hybrid, as shown in the fluorescence photos (inset of Fig. 3a). The slight blue shift of the emission peak (from 462 nm to 452 nm) with [Cr3+] might be attributed to the disappearance of the size-dependent trap states of the N-doped-CDs@GNSs hybrid.5a This suggested that the N-doped-CDs@GNSs hybrid might be partially deconstructed by Cr3+, as a result of the stronger affinity between Cr3+ and the GNSs, which will be discussed in detail in the following part. Therefore, the as-prepared N-doped-CDs@GNSs hybrid can be used for fluorescence “turn on” detection of Cr3+. For a sensitivity study, different concentrations of Cr3+ in the range of 0–5 × 103 nM were investigated. As depicted in Fig. 3b, the fluorescence intensity (I) of the N-doped-CDs@GNSs hybrid solution increases steadily with [Cr3+]. According to our experiments, for 5 × 10−6 M Cr3+, the fluorescence quantum yield of the N-doped-CDs@GNSs hybrid was increased to 5% using rhodamine 6G as a reference. The linear response range is shown in the inset of Fig. 3b, where the ratio of the fluorescence intensities (I/I0) vs. [Cr3+] can be curve-fitted to
| I/I0 = 2.05 + 2.4 × 108[Cr3+], |
Selectivity is an important parameter to evaluate the performance of a fluorescence sensing system. Therefore, the fluorescence responses of the N-doped-CDs@GNSs hybrid to representative common metal ions, including Na+, K+, Mg2+, Ca2+, Al3+, Cd2+, Cu2+, Hg2+, Co2+, Ni2+, Pb2+ and Zn2+, were examined. As shown in Fig. 3c, it can be seen that much higher fluorescence intensity was observed for the N-doped-CDs@GNSs hybrid with Cr3+. In contrast, no fluorescence enhancement was observed for the addition of any other metal ions mentioned above. Furthermore, as shown in Fig. 3d, the present N-doped-CDs@GNSs hybrid can achieve fluorescence “turn on” detection of Cr3+ in the presence of any of the above mentioned interference metal ions (the concentration of each metal ion in the mixture was 500 nM). Specifically, the existence of Cr2O72− has negligible interference to the detection of Cr3+ even when its concentration reaches 10−5 M (Fig. S3†), thus the N-doped-CDs@GNSs hybrid can be used for selective fluorescence “turn on” detection of Cr(III). It is further proved that the fluorescence intensity of the N-doped-CDs@GNSs hybrid is stable under high ionic strength conditions (Fig. S4a†) but strongly depends on the pH value of the solution (Fig. S4b†), being similar to the previous report.1a The fluorescence intensity of the N-doped-CDs@GNSs hybrid reaches highest at pH 7 (Fig. S4a†), suggesting their potential applications in physiological conditions.
For practical application, the feasibility of the N-doped-CDs@GNSs hybrid for detecting Cr3+ in real water samples was explored, by using tap water and lake water samples obtained from the Shushan Lake of Hefei, Anhui Province, China. The lake water sample was filtered through common filter papers before further use, while the tap water sample was used without any further treatments. Both of the water samples were spiked with standard solutions containing known concentrations of Cr3+. As shown in Fig. 4, it can be seen that for both the tap water and the lake water samples, the fluorescence intensity of the N-doped-CDs@GNSs hybrid increases with [Cr3+]. The approximate calibration curve for determining Cr3+ in tap water and lake waters were obtained by plotting the values of I/I0 versus [Cr3+] (Fig. 4b and d). Although there exists interference from numerous compounds, silts and organics existing in the tap water and lake waters, the as obtained N-doped-CDs@GNSs can still detect 7 nM (10 times of the detection lower limit in DI-water) Cr3+ for both the tap water and the lake water samples, which is sensitive enough for the practical Cr3+ detection in real samples. Taken together, the obtained N-doped-CDs@GNSs hybrid might have potentials for the detection of trace Cr3+ in biological, medical and environmental fields.
 | ||
| Fig. 4 Fluorescence spectra and corresponding linear relationships between I/I0 and [Cr3+] for contaminated (a and b) tap water and (c and d) lake water samples. | ||
In order to study the sensing mechanism of the N-doped-CDs@GNSs hybrid to Cr3+, products with different N-doping amounts were prepared, and their fluorescence responses to common metal ions were studied (Fig. S5 in the ESI†). For the product obtained without 1,6-hexamethylenediamine (denoted as product 1), both Cu2+ and Pb2+ can quench its fluorescence (Fig. S5a†), and no obvious quenching or enhancement occurs for other metal ions including Cr3+. For the product obtained with 0.8 ml 1,6-hexamethylenediamine in the hydrothermal process (denoted as product 2), however only Cu2+ can quench its fluorescence (Fig. S5b†), thus product 2 can be used for fluorescence “turn off” detection of Cu2+ (Part S2 and Fig. S6 in the ESI†). When the amount of 1,6-hexamethylenediamine is increased to 1 ml (denoted as product 3), slight fluorescence enhancement induced by Cr3+ is firstly observed, but there still exists slight fluorescence quenching induced by Cu2+ (Fig. S5c†). When the amount of 1,6-hexamethylenediamine is further increased to 2 ml (denoted as product 4), selective Cr3+ fluorescence enhancement is realized (Fig. S5d†). However, for the product obtained using 1,6-hexamethylenediamine alone (without peanut shells as raw material, denoted as product 5), emission can be quenched by both Cu2+ and Pb2+ (Fig. S5e†). It should be noted that the existence of NaOH was not essential for the selectivity of the products to specific heavy metal ions (Fig. S5f†). Furthermore, our Inductively Coupled Plasma (ICP) experiments proved that the N-doped-CDs@GNSs hybrid had superior adsorbability to Cr3+ compared to other metal ions used (see Fig. S7†). However, product 2 showed good adsorption to Cu2+, while product 1 and product 5 showed adsorbability to both Cu2+ and Pb2+. These differences might be attributed to the different kinds and different amounts of chemical groups formed on the surface of the products.
To further study the interaction mechanism of the N-doped-CDs@GNSs hybrid with Cr3+, UV-vis spectra were taken (see Fig. S8†). It was proved that the free N-doped-CDs@GNSs hybrid shows two absorption peaks at 280 nm and 350 nm respectively, with the former being attributed to the π–π* transition absorption of aromatic sp2 domains of the N-doped-CDs,4a,17 while the latter at 350 nm attributed to the absorption of the large sized N-doped-CDs@GNSs hybrid.5a With the successive addition of Cr3+, the absorption peak at 350 nm (corresponding to the absorption of the N-doped-CDs@GNSs hybrid) decreases accompanied with the increase of that at 280 nm (corresponding to the π–π* transition absorption of the N-doped-CDs). All of the above-mentioned result indicates that the presence of Cr3+ has partially deconstructed the N-doped-CDs@GNSs hybrid and increased the amount of free N-doped-CDs, due to the stronger affinity between Cr3+ and the GNSs. Thus the fluorescence of the N-doped-CDs@GNSs hybrid system recovers in a [Cr3+] dependent manner. To further study the interaction between Cr3+ and the GNSs, XPS spectra of the N-doped-CDs@GNSs hybrid before and after interaction with Cr3+ were measured (see Fig. S9 in the ESI†). And it was shown that there exists obvious peak shifts in the N 1s and O 1s spectra of the N-doped-CDs@GNSs hybrid after interaction with Cr3+, proving that the GNSs interacts with Cr3+ mainly via nitrogen and oxygen groups on the surface of the GNSs. Furthermore, this interaction may also be related to the synergistic effect of the GNSs.15 As a result of this kind of specific interaction between the GNSs and Cr3+, a part of the N-doped-CDs were broken away from the GNSs, thus FRET process from the excited N-doped-CDs to the GNSs is seriously inhibited, and the emission of the N-doped-CDs@GNSs hybrid is greatly enhanced.
Conclusions
In summary, we present a simple and cheap fabrication strategy for N-doped-CDs@GNSs hybrid using waste peanut shells and 1,6-hexamethylendiamine as raw materials, and the resultant N-doped-CDs@GNSs hybrid has been used for selective fluorescence “turn on” sensing of Cr3+ with a lower detection limit of 0.7 nM. Furthermore, the N-doped-CDs@GNSs hybrid has been successfully used for analysis of Cr3+ in real tap water and real lake water samples, demonstrating their potential applications as practical environmental probes. The sensing mechanism is ascribed to the competition between Cr3+ and the N-doped-CDs on the surface of the GNSs, which partially inhibits FRET from the excited N-doped-CDs to GNSs. The present study shows a new avenue to design fluorescence “turn on” sensors for heavy metal ions based on CDs.Acknowledgements
This work was financially supported by the National Key Basic Research Program of China (Grant 2013CB934304), the NSFC (21307138 and 51472245) and SRG-HSC. Research Supported by the CAS/SAFEA International Partnership Program for Creative Research Teams.Notes and references
- (a) W. Lu, X. Qin, S. Liu, G. Chang, Y. Zhang, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Anal. Chem., 2012, 84, 5351–5357 CrossRef CAS PubMed; (b) Y. Fu, C. Ding, A. Zhu, Z. Deng, Y. Tian and M. Jin, Anal. Chem., 2013, 85, 11936–11943 CrossRef CAS PubMed; (c) I. Costas-Mora, V. Romero, I. Lavilla and C. Bendicho, Anal. Chem., 2014, 86, 4536–4543 CrossRef CAS PubMed; (d) X. Gao, C. Ding, A. Zhu and Y. Tian, Anal. Chem., 2014, 86, 7071–7078 CrossRef CAS PubMed; (e) X. Liu, N. Zhang, T. Bing and D. Shangguan, Anal. Chem., 2014, 86, 2289–2296 CrossRef CAS PubMed; (f) C. Yuan, B. Liu, F. Liu, M. Han and Z. Zhang, Anal. Chem., 2014, 86, 1123–1130 CrossRef CAS PubMed; (g) M. J. Ruedas-Rama and E. A. H. Hall, Anal. Chem., 2008, 80, 8260–8268 CrossRef CAS PubMed; (h) Z. Wang and S. Ding, Anal. Chem., 2014, 86, 7436–7445 CrossRef CAS PubMed; (i) A. Ananthanarayanan, X. Wang, P. Routh, B. Sana, S. Lim, D. Kim, K. Lim, J. Li and P. Chen, Adv. Funct. Mater., 2014, 24, 3021–3026 CrossRef CAS; (j) G. E. LeCroy, S. K. Sonkar, F. Yang, L. Monica Veca, P. Wang, K. N. Tackett, J. Yu, E. Vasile, H. Qian, Y. Liu, P. Luo and Y. Sun, ACS Nano, 2014, 8, 4522–4529 CrossRef CAS PubMed; (k) S. Liu, J. Tian, L. Wang, Y. Zhang, X. Qin, Y. Luo, A. M. Asiri, A. O. Al-Youbi and X. Sun, Adv. Mater., 2012, 24, 2037–2041 CrossRef CAS PubMed; (l) H. Zhang, Y. Li, X. Liu, P. Liu, Y. Wang, T. An, H. Yang, D. Jing and H. Zhao, Environ. Sci. Technol. Lett., 2014, 1, 87–91 CrossRef CAS.
- M. Zheng, Z. Xie, D. Qu, D. Li, P. Du, X. Jing and Z. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 13242–13247 CAS.
- W. Kong, R. Liu, H. Li, J. Liu, H. Huang, Y. Liu and Z. Kang, J. Mater. Chem. B, 2014, 2, 5077–5082 RSC.
- (a) H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. A. Tsang, X. Yang and S. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430–4434 CrossRef CAS PubMed; (b) T. Yeh, C. Teng, S. Chen and H. Teng, Adv. Mater., 2014, 49, 3297–3303 CrossRef PubMed.
- (a) H. Zhang, Y. Wang, D. Wang, Y. Li, X. Liu, P. Liu, H. Yang, T. An, Z. Tang and H. Zhao, Small, 2014, 10, 3371–3378 CrossRef CAS PubMed; (b) L. Shen, Q. Chen, Z. Sun, X. Chen and J. Wang, Anal. Chem., 2014, 86, 5002–5008 CrossRef CAS PubMed.
- (a) P. Roy, A. P. Periasamy, C. Chuang, Y. Liou, Y. Chen, J. Joly, C. Liang and H. Chang, New J. Chem., 2014, 38, 4946–4951 RSC; (b) S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed; (c) L. Mao, W. Tang, Z. Deng, S. Liu, C. Wang and S. Chen, Ind. Eng. Chem. Res., 2014, 53, 6417–6425 CrossRef CAS.
- L. Wang, S. Zhu, H. Wang, S. Qu, Y. Zhang, J. Zhang, Q. Chen, H. Xu, W. Han, B. Yang and H. Sun, ACS Nano, 2014, 8, 2541–2547 CrossRef CAS PubMed.
- M. F. Chang, I. R. Ginjom, M. Ngu-Schwemlein and S. M. Ng, Microchim. Acta, 2016, 183, 1899–1907 CrossRef CAS.
- L. Liu, Y. Li, L. Zhan, Y. Liu and C. Huang, Sci. China: Chem., 2011, 54, 1342–1347 CrossRef CAS.
- M. Wang, G. Meng, Q. Huang and Y. Qian, Environ. Sci. Technol., 2012, 46, 367–373 CrossRef CAS PubMed.
- J. Mao, Q. He and W. Liu, Anal. Bioanal. Chem., 2010, 396, 1197–1203 CrossRef CAS PubMed.
- Y. Wan, Q. Guo, X. Wang and A. Xia, Anal. Chim. Acta, 2010, 665, 215–220 CrossRef CAS PubMed.
- L. Zhang, C. Xu and B. Li, Microchim. Acta, 2009, 166, 61–68 CrossRef CAS.
- Y. Wan, Q. Guo, X. Wang and A. Xia, Anal. Chim. Acta, 2010, 665, 215–220 CrossRef CAS PubMed.
- W. Wei, C. Xu, J. Ren, B. Xu and X. Qu, Chem. Commun., 2012, 48, 1284–1286 RSC.
- M. Wang, G. Meng, Q. Huang, Y. Lu and Y. Gu, Sens. Actuators, B, 2013, 185, 47–52 CrossRef CAS.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Other details and additional figures. See DOI: 10.1039/c6ra14732a |
| This journal is © The Royal Society of Chemistry 2016 |