Effect of char structures caused by varying the amount of FeCl3 on the pore development during activation†
Dongdong Liu,
Jihui Gao*,
Shaohua Wu and
Yukun Qin
College of Energy Science and Engineering, Harbin Institute of Technology, Harbin 150001, China. E-mail: gaojh@hit.edu.cn; Fax: +86 451 8641 2528; Tel: +86 451 8641 3231
First published on 29th August 2016
Abstract
A series of Fe-loaded coal chars is obtained by varying the load amount of FeCl3 additives (0, 6, 15 and 30 wt%). The effect of FeCl3 on the char structure during pyrolysis is presented; furthermore, the chars with different typical structures are activated under different burn-offs to study the pore development during activation. The results show that the mesopore size distribution is related to the amount of FeCl3. A small amount of FeCl3 additive (6 and 15 wt%) can promote depolymerization of the aromatic structure and formation of well-developed spatial crosslinks, and a greater amount of FeCl3 additive (30 wt%) can accelerate the graphitization tendency. The pore formation of 0Fe-900 without initial mesopores follows a hierarchical development from the surface to the core. The chars 15Fe-900H, 15Fe-900H and 30Fe-900H with some initial mesopores follows a non-hierarchical development from the external and internal particles simultaneously; however, the difference in microstructure can affect the penetration of the activated agent into the particle, resulting in different burn-offs and external carbon losses when the hierarchical porous structure has been produced.
1 Introduction
Activated carbon (AC) is often used as an adsorbent material in the environmental field.1 The hierarchical porous structure (micropores and mesopores) of AC is of great importance in the removal of gas-phase pollutants (such as SO2). Sun et al.2 and Shu et al.3 reported that micropores act as adsorption sites for SO2 adsorption and catalysis reactions and subsequently concluded that a well-developed mesopore is conducive to the migration and storage of sulfuric acid to create adsorption sites within the micropores. Furthermore, Rubio et al.4 and Lizzio et al.5 showed that specific surface area (SBET) can directly reflect the degree of well-developed pores, which is proportional to the saturated sulfur capacity of activated carbon.At present, a wider application of AC is greatly limited by its high cost. Zhu et al.6,7 have shown that the hierarchical structure of AC can be clearly formed, but only if the burn-off exceeds 50%; at this burn-off value, an excessive loss of quality at the particles' surfaces has been found, and it increases the total amount of carbon loss but does not form porous structures. A specific analysis is as follows: the diffusion of the activated agent (such H2O and CO2) and activated product (such H2 and CO) is hindered by the coal char structure that is produced by pyrolysis with a large amount of ordered microcrystalline, poorly-developed spatial crosslinks, and fewer initial pores.8,9 Several researchers10–12 have attempted to obtain a wide pore distribution of AC using different methods such as the blending method and supercritical water activation; however, the problem of the excessive loss of particle surface still cannot be fundamentally resolved. Therefore, a typical char structure containing a higher number of initial mesopores, disordered microcrystalline and well-developed spatial crosslinks should be prepared to modify the pore development during activation. Such a method could aid in the development of a hierarchical pore structure of AC at lower burn-offs.
To obtain the typical char structure mentioned above, Fe-based compounds are used for the following reasons. First, Fe-based compounds (such as Fe2O3 and FeCl3) can serve as templates to synthesize mesoporous materials.13,14 A mixture containing raw coal and metal compound is heated to high temperatures. After pyrolysis, the metal compounds are easily removed from coal chars using diluted hydrochloric acid, and subsequently, a clear mesoporous structure is obtained. Second, Fe-based compounds can also improve the structure of the microstructures. Xu et al.15 and Oztas et al.16 showed that Fe-based compounds can catalyse the decomposition of all hydrocarbons within chars except methane. This reaction promotes the generation and release of volatiles and hinders the condensation of free radicals during pyrolysis. Finally, iron is one of the common components in coal and has a lower cost than any other metal such as vanadium, nickel, and zirconium. Therefore, the preparation of a typical char during pyrolysis can be carried out by varying the number of Fe-based compounds. These play an important role in determining the pore development during activation, but they have rarely been systematically discussed.
In this study, FeCl3 was used as an additive to prepare a series of Fe-loaded chars with different macroscopic and microscopic structures to study the effects that various amounts of FeCl3 have on the structure of coal chars during pyrolysis. Then, by comparing the relationship between carbon loss and pore formation for chars with a typical structure, the pore development was rigorously investigated during activation. The characterization of resultant samples was investigated by scanning electron microscopy (SEM), nitrogen adsorption, X-ray diffraction (XRD) and Raman spectroscopy.
2 Experimental section
2.1 Sample preparation
Jixi bituminous coal from North China was used as the raw coals and were crushed and sieved to particles of 250–380 μm in size. The raw coals were washed with deionized water, and then dried at 150 °C in nitrogen for 20 h to remove water and stored in a glass bottle before use. In accordance with standard methods,17 the demineralization procedure of the coal samples was performed as follows: 150 mL of 6 mol L−1 HCl was added to 3 g of coal samples and stirred for 12 h at 40 °C. Then, the mixture was filtered and mixed with 120 mL of 40% HF and stirred again at for 12 h at 40 °C. After filtration, the mixture was washed with deionized water to remove chloride ions. Finally, the acid-treated coal sample was dried at 80 °C in nitrogen for 20 h. Data from proximate and ultimate analyses of acid-treated samples are given in Table 1; the content of ash in the acid-treated sample was below 1% is and denoted as JX.The procedure for catalyst addition was conducted in the following manner: After a predetermined amount of FeCl3 powder (0.09 g, 0.18 g, 0.45 g and 0.9 g, respectively) and 3 g of acid-treated coals (JX) were added to an aqueous solution, the resulting mixture was stirred for 24 h in a sealed beaker at ambient temperature, evaporated under vacuum and dried at 80 °C overnight before the pyrolysis experiment. This method has made it possible to incorporate finely dispersed FeCl3 into the coal sample.
2.2 Experimental process
(1) The experimental pyrolysis procedure was conducted in the following manner. Fe-loaded samples were placed in a three-stage tube furnace reactor at a fixed heating rate of 8 °C min−1 from room temperature to 900 °C and maintained for 1 h under an N2 atmosphere, as illustrated in Fig. 1. Then, these samples were quenched to room temperature under an N2 atmosphere and denoted as 6Fe-900, 15Fe-900 and 30Fe-900, respectively. After pyrolysis, these samples were treated by 0.2 mol L−1 HCl and washed with distilled water to remove chloride ions. Finally, the samples were dried at 60 °C in nitrogen for 12 h and denoted as 6Fe-900H, 15Fe-900H and 30Fe-900H, respectively. A comparative sample without adding FeCl3 was prepared by the same pyrolysis condition and denoted as 0Fe-900.(2) The experimental activation procedure was conducted in the following manner. These char samples were held continuously for an appropriate time at 900 °C under a constant flow of CO2 and quenched to room temperature under a N2 atmosphere to obtain AC with different burn-offs and porous structures. These AC samples were denoted as (0Fe-900, 6Fe-900H, 15Fe-900H and 30Fe-900H)-burn-off rates.
2.3 Measurement analysis
The carbonized char powder samples were investigated using a D/max-rb X-ray diffractometer. The samples were measured in the 2θ range from 5° to 85° using a scan rate of 3° min−1 to obtain the structural information regarding the crystallite of the samples. The HCl-washed carbonized char was examined using Raman spectroscopy at a 532 nm wavelength. The Raman spectra data were carefully acquired in the range of 1000–1800 cm−1, including first-order bands. Transmission electron microscopy (TEM, Tecnai G2 F30) was operated at 200 kV to visualize the structural changes. Nitrogen adsorption isotherms were obtained using a Micromeritics Adsorption Apparatus (ASAP2020) at 77 K to obtain pore structure information of the samples. The BET surface area (SBET) was obtained using the BET (Brunauer–Emmett–Teller) equation in the relative pressure range from 0.05 to 0.24. The total pore volume (Vt) was obtained at a relative pressure of 0.98. The pore size distribution (PSD) in the micropore and mesopore range was calculated using the Horvath–Kawazoe method and the Barrett–Joyner–Halenda (BJH) method, respectively. Moreover, PSD was also calculated by the density functional theory (DFT).3 Results and discussion
3.1 TEM analysis of coal chars
Fig. 2 shows several TEM images of four chars at a high resolution. For 6Fe-900, 15Fe-900 and 30Fe-900 in Fig. 2(a)–(c), the spherical- or a ball-like particles encapsulated by multi-layer amorphous carbons can be found near a number of long parallel-aligned crystallite layers having different orientations. Other crystallites and amorphous carbon, quite unlike these carbon-coated iron particles, are stacked disorderedly. In addition, as the amount of FeCl3 additive increases from 6 to 30 wt%, these nanoparticles begin to move and gradually blend with each other. This leads to an increase in their diameter from 7 nm to 20 nm. However, there are no carbon-coated iron particles for 0Fe-900 in Fig. 2(d), only an array of amorphous carbon and crystallites that are randomly distributed.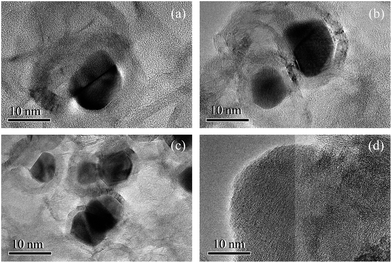 | ||
| Fig. 2 TEM images from different chars with/without the additives (a) 6Fe-900, (b) 15Fe-900, (c) 30Fe-900, (d) 0Fe-900. | ||
According to the literature18–20 regarding the mechanism of metal-catalyzed graphitization, the formation of iron carbides (such as FeC3) first originates from the reaction between iron and amorphous carbon at high pyrolysis temperatures. Then, the formation of graphitized carbon is attributed to the decomposition of these metal carbides under certain conditions. Finally, the entire reaction process can be expressed as follows:
| α-Fe(s) + amorphous carbon(s) → Fe3C(s) | (1) |
| Fe3C(s) → α-Fe(s) + graphitized carbon(s) | (2) |
3.2 Pore structure characterization of coal chars
Fig. 3(a) shows the N2 adsorption isotherms for four chars. First, the N2 adsorption capacities of 0Fe-900 are extremely small, indicating few pores. Second, the N2 adsorption isotherms of 6Fe-900H, 15Fe-900H and 30Fe-900H are related to type I at low pressures and type IV at high pressures, according to the IUPAC classification. However, these isotherms do not level off and begin to branch, and a hysteresis loop can be observed as the relative pressure continues to increase. With the increase of FeCl3 additive from 6 to 30 wt%, the adsorption capacities increase slightly at low pressures and the isotherms become steeper at high pressures, indicating the formation of some microporous and a greater amount of mesoporous structure. Gong et al.21 proved that more volatile matter (such as CO or CO2) is released by adding Fe-based compounds to form micropores.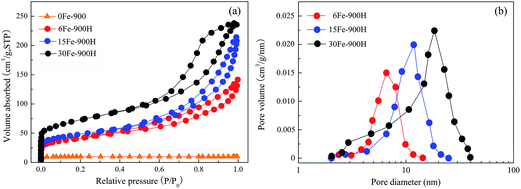 | ||
| Fig. 3 (a) N2 adsorption/desorption isotherms and (b) the corresponding pore size distribution of different chars with/without the additives. | ||
Fig. 3(b) shows the pore-size distribution determined by the BJH method for 6Fe-900H, 15Fe-900H and 30Fe-900H. The size distribution of their mesopores is relatively broad, where the size ranges from 2 to 40 nm as the amount of FeCl3 additive is increased. The average size of the mesopores is consistent with the diameter size of the carbon-coated iron particles shown in Fig. 2; therefore, the mesopores are formed by removing the nanoparticles that occupy the inner space of the coal chars. As the amount of FeCl3 addition is increased from 6 to 30 wt%, the pore-size distribution of the mesopores gradually increases. This may be related to a greater concentration of carbon-coated iron particles at high temperatures. According to Ma et al.,22 more and more minerals have been found that aggregate to form the spheres inside chars at high temperatures with the spheres still containing a certain amount of carbon.
3.3 Phase and crystal structure of coal chars by XRD
Fig. 4 shows the XRD profiles for 0Fe-900, 6Fe-900H, 15Fe-900H and 30Fe-900H. The two marked broad diffraction peaks of (002) at 2θ = 24–27° and (100) peak at 2θ = 41–44°, which correspond to an interplanar spacing dependent upon the stacking of planes and the degree of condensation in the aromatic rings, are observed for all samples.23 According to Zhang et al.,24 several parameters must be obtained from the XRD profiles using the Origin 9.1 software to determine the peak fitting treatment, as shown in Fig. 5; the aromatic structure layer distance (d002), stacking height (Lc), width (La) and the number of aromatic layers (N = Lc/d002) are shown in Table 2.| 0Fe-900 | 6Fe-900H | 15Fe-900H | 30Fe-900H | |
|---|---|---|---|---|
| La (Å) | 25.856 | 22.161 | 20.081 | 31.181 |
| Lc (Å) | 13.909 | 13.550 | 13.075 | 15.458 |
| d002 | 3.557 | 3.613 | 3.704 | 3.142 |
| N | 3.91 | 3.75 | 3.53 | 4.92 |
As shown in Table 2, the values of La, Lc and N decrease, compared with the parameters of 0Fe-900, and d002 increases gradually as the amount of FeCl3 increases from 6 to 15 wt%. However, when the addition of FeCl3 reaches 30 wt%, these parameters reverse their variation tendency. These results show that a small amount of FeCl3 additive (6 and 15 wt%) with a better dispersion and mobility may penetrate into an aromatic structure to enlarge the distance between the layers. This reaction promotes structural depolymerization, resulting in a transformation towards a non-graphite carbon form. On the other hand, a greater amount of the FeCl3 additive (30 wt%) facilitates the rapid growth and enhances order in the aromatic structure, resulting in graphitization.
3.4 Carbon structure of coal chars by Raman
Fig. 6 shows the Raman spectra for 0Fe-900, 6Fe-900H, 15Fe-900H and 30Fe-900H. According to the studies by Li et al.,25 the assignment of five bands in the Raman spectra of chars is summarized in Table 3. The band area ratios of the D1 band corresponding to the G band (ID1/IG) can be described as the defect degree of the crystallite structure. The ratio of the D3 bands to the G band (ID3/IG) is extremely useful to study the representative structures of amorphous carbon, and the ratio of the D4 bands to the G band (ID4/IG) is also regarded as the primary measure to describe the cross-linking density. After spectral normalization treatment, the total spectral area (Iall) can also be regarded as the primary measure of char reactivity.26–28 The software, Origin 9.1, is used for the spectrogram smoothness, baseline correction and normalization for all samples, as shown in Fig. 7. The different band area ratios are shown in Table 4.| Band name | Band position (cm−1) | Description | Bond type |
|---|---|---|---|
| D2 | 1620 | Graphite E22g; carbonyl group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
sp2 |
| G | 1590 | Graphite E22g; aromatic ring quadrant breathing; alkene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
sp2 |
| D3 | 1530 | Aromatics with 3–5 rings; semi-circle breathing of aromatic rings; methylene or methyl; amorphous carbons structures | sp2, sp3 |
| D1 | 1350 | C–C between aromatic rings and aromatics with not less than 6 rings; introduced disorder carbon | sp2 |
| D4 | 1200 | Caromatic–Calkyl; aromatic (aliphatic) ethers; C–C on hydroaromatic rings; hexagonal diamond carbon sp3; C–H on aromatic rings | sp2, sp3 |
| 0Fe-900 | 6Fe-900H | 15Fe-900H | 30Fe-900H | |
|---|---|---|---|---|
| AD1/AG | 3.246 | 3.441 | 3.736 | 1.896 |
| AD3/AG | 1.942 | 2.189 | 3.729 | 0.895 |
| AD4/AG | 0.617 | 0.701 | 0.799 | 0.581 |
| Aall | 313.03 | 332.69 | 356.74 | 250.08 |
As shown in Table 4, the values of ID1/IG, ID3/IG, ID4/IG and Iall increase gradually for 6Fe-900H and 15Fe-900H, compared with the parameters of 0Fe-900. As the amount of FeCl3 additive increases from 3 to 15 wt%, the change in 15Fe-900H is much larger than that of 6Fe-900H. A low amount of FeCl3 additive (6 and 15 wt%) facilitates the splitting of the macromolecular structure during pyrolysis, resulting in the transformation of the crystalline sp2 structure (G peak) into the isolated and defective sp2 structure (D1 peak) and the production of more amorphous carbon (D3 peak). This promotes an increase in the disorder and high char reactivity. An increase in the cross-linking density (D4 peak) is responsible for the penetration of the iron atoms into the crystallite structure to form a better developed three-dimensional structure. However, the abrupt diminution in all four parameters for 30Fe-900H can also be clearly observed, indicating that a greater amount of FeCl3 additive (30 wt%) accelerates the graphitization tendency of the aromatic structure, leading to the transformation of the defective sp2 structure and amorphous sp2–sp3 structure into the crystalline sp2 structure, and the disruption of cross-linking bonds (sp3-rich structures), indicating the beginning of graphitization.
3.5 Pore structure characterization of coal chars during activation
To further investigate the pore development during activation, the relationship between carbon loss and pore formation for different precursor chars (0Fe-900, 15Fe-900H and 30Fe-900H) with typical structures was compared. This decision was based on the previously presented microstructure and macrostructure data analysis of these chars during pyrolysis. The N2 adsorption isotherms, the pore-size distribution and parameters of porous structure are shown in Fig. 8 and Table 5. | ||
| Fig. 8 N2 adsorption isotherms and pore-size distribution of different chars during activation (a) and (b) 0Fe-900, (c) and (d) 6Fe-900H, (e) and (f) 15Fe-900H, (g) and (h) 30Fe-900H. | ||
| Samples | SBETa (m2 g−1) | Vtb (m3 g−1) | Vmicc (m3 g−1) | Non-Vmicd (%) | Dave (nm) |
|---|---|---|---|---|---|
| a Specific surface area determined by the BET method for P/P0 from 0.05 to 0.24.b Total pore volume calculated at P/P0 = 0.98.c Volume of micropores (<2 nm) calculated by the t-plot method.d Non-Vmic, Vt minus Vmic (>2 nm).e Average pore diameter (Dav = 4Vt/SBET). | |||||
| 0Fe-900-12.1 | 119.71 | 0.075 | 0.071 | 4.86 | 2.51 |
| 0Fe-900-29.5 | 325.76 | 0.154 | 0.137 | 11.19 | 1.89 |
| 0Fe-900-55.2 | 658.53 | 0.316 | 0.254 | 19.79 | 1.92 |
| 6Fe-900-12.8 | 258.36 | 0.208 | 0.118 | 43.26 | 3.22 |
| 6Fe-900-40.1 | 786.25 | 0.340 | 0.286 | 15.88 | 1.73 |
| 15Fe-900H-15.9 | 384.85 | 0.341 | 0.101 | 70.67 | 3.91 |
| 15Fe-900H-31.2 | 849.37 | 0.414 | 0.355 | 14.25 | 1.94 |
| 30Fe-900H-14.8 | 105.63 | 0.582 | 0.101 | 82.64 | 11.32 |
| 30Fe-900H-50.6 | 308.14 | 0.639 | 0.211 | 66.98 | 8.29 |
From Fig. 8(a) and (b), the N2 adsorption isotherm of 0Fe-900-12.1 is clearly of type I, indicating the typical microporous structure with a narrow size distribution of less than 1 nm. Then, the isotherm capacities of 0Fe-900-29.5 at a low pressure are enhanced, and the isotherm gradually becomes steep with an increase in the relative pressure; a slight hysteresis loop is also observed at high pressures. This reaction results in an increase of the number of micropores and the formation of some mesopores (2–4 nm). The N2 isotherm of 0Fe-900-55.2 is related to type I at low pressures and type IV at high pressures, showing the obvious hierarchical porous structure. However, serious carbon loss at the surface of the particle has been found in Fig. S1.†
In Fig. 8(c) and (d), the N2 adsorption isotherms of 15Fe-900H-15.9 and 15Fe-900H-31.2 are all of type I at low pressure and of type IV at high pressures. As the burn-offs increase from 15.9% to 31.2%, there is an obvious increase in the isotherm capacities at a low pressure, but only a slight change at a high pressure, showing that the quality loss is primarily associated with the micropore formation during the entire activation. The char 15Fe-900H-31.2 formed an obvious hierarchical porous structure and a high value in SBET (849.37 m2 g−1), and some pores are also found at the particle surfaces in Fig. S1.† In addition, the change in the porous structure of 6Fe-900H, as shown in from Fig. 8(e) and (f), is similar to that of 15Fe-900H due to their similar structures. As the burn-offs increase from 12.8% to 40.1%, the isotherm capacities at low pressures gradually increase. This may be related to its developed degree of spatial crosslinking structure, but no serious carbon losses at the particle surfaces in Fig. S1.†
In Fig. 8(g) and (h), as the burn-offs increase from 14.8% to 50.6% for 30Fe-900H, only a few increases in the isotherm capacities at low pressure are noted, showing the slow growth of the micropores. Serious carbon loss at the particles' surfaces is shown in Fig. S1† when 30Fe-900H-50.6 contains the hierarchical porous structure but a low value in SBET (308.14 m2 g−1).
3.6 Mechanism of pore development during activation
Based on the abovementioned analysis about carbon loss and pore formation for different chars during activation, the different pore development is shown in Fig. 9.The char 0Fe-900 has fewer initial pores, resulting in the successive diffusion of the activated agent from the surface to the core at the beginning of the activation. Owing to the greater amounts of ordered microcrystalline and poorly-developed spatial crosslinks for 0Fe-900, the penetration of the activated agent into the particle will decrease and the reaction will mainly occur outside the particle; thus, its surface was burned severely when the hierarchical porous structure is formed at high burn-off of 55.2%. Therefore, the pore formation of 0Fe-900 follows a hierarchical development.
The chars 6HFe-900H, 15Fe-900H and 30Fe-900H have initial mesopores to reduce the diffusion limitation of the activated agent and products, resulting in simultaneous diffusion of the activated agent during activation of the external and internal particles, but the difference in microstructure, such as spatial crosslinks and ordered degree of crystallites, can also affect the development of subsequent porous structures. The well-developed spatial structures and highly disordered crystallites of 15Fe-900H can generate a large amount of micropores quickly, resulting in the hierarchical porous structure being formed at low burn-off of 31.2% with no excessive carbon loss at the particle surface. Moreover, the pore development of 6Fe-900H is similar to 15Fe-900H during activation, but micropore volume (0.286 m3 g−1) and SBET (786.25 m2 g−1) of 6Fe-900H-40.1 are lower than that (0.355 m3 g−1, 849.37 m2 g−1) of 15Fe-900H-31.2. However, the poorly developed spatial crosslinks and highly ordered crystallites for 30Fe-900H hinder the reaction between the activated agent and the carbon structure, finally resulting in the slow growth of micropores and the serious external loss at the particles' surfaces. Therefore, the pore formation of 6HFe-900H, 15Fe-900 and 30Fe-900 follows a non-hierarchical development.
4 Conclusions
The chars with typical structures were prepared by varying the loaded amount of FeCl3 additive and the pore development of different chars was also studied.(1) The increase in the initial mesopores size of coal chars is related to the increase in the amount of FeCl3 additive from 6 to 30 wt%. As the amount of FeCl3 additive increases from 6 to 15 wt%, the crystallite structure starts out only slightly graphitized, but a higher amount of FeCl3 additive (30 wt%) can accelerate the graphitization tendency.
(2) The pore formation of 0Fe-900 without initial mesopores follows a hierarchical development from the surface to the core, but the more ordered microcrystalline and poorly-developed spatial crosslinks hinder the penetration of the activated agent into the particle, resulting in the formation of hierarchical pores at high burn-off of 55.2% and severe carbon loss at the particles' surfaces.
(3) The pore formation of 6Fe-900H, 15Fe-900H and 30Fe-900H follows a non-hierarchical development during simultaneous activation of the external and internal particles, but 6Fe-900H and 15Fe-900H with a well-developed spatial structure and highly disordered crystallites have the hierarchical pores at lower burn-offs of 31.2% and 40.1%, respectively, showing the rapid development of micropores with no serious external loss at the particles' surfaces. However, 30Fe-900H with the poorly developed spatial crosslinks and highly ordered crystallites form hierarchical pores at high burn-off of 50.6% and a low value of SBET (308.14 m2 g−1), showing the slow growth of micropores and the serious external loss at the particles' surfaces.
Acknowledgements
The authors greatly thank the financial support for this research project from the National Natural Science Foundation of China (51276052).Notes and references
- Q. Y. Liu and Z. Y. Liu, Fuel, 2013, 108, 149–158 CrossRef CAS.
- F. Sun, J. H. Gao, X. Liu, X. F. Tang and S. H. Wu, Appl. Surf. Sci., 2015, 357, 1895–1901 CrossRef CAS.
- S. Shu, J. X. Guo, X. L. Liu, X. J. Wang, H. Q. Yin and D. M. Luo, Appl. Surf. Sci., 2016, 360, 684–692 CrossRef CAS.
- B. Rubio and M. T. Izquicrdo, Fuel, 1998, 77, 631–637 CrossRef CAS.
- A. A. Lizzio and J. A. Debarr, Fuel, 1996, 75, 1515–1522 CrossRef.
- Y. W. Zhu, J. H. Gao, Y. Li, F. Sun, J. M. Gao, S. H. Wu and Y. K. Qin, J. Taiwan Inst. Chem. Eng., 2012, 43, 112–119 CAS.
- Y. W. Zhu, J. H. Gao, Y. Li, F. Sun and Y. K. Qin, Korean J. Chem. Eng., 2011, 28, 2344–2350 CrossRef CAS.
- X. X. Zhou, X. Qu, R. Zhang and J. C. Bi, Fuel Process. Technol., 2015, 135, 195–202 CrossRef CAS.
- P. L. Walker, Carbon, 1996, 34, 1297–1299 CrossRef CAS.
- Z. Q. Wu, S. Z. Wang, J. Zhao, L. Chen and H. Y. Meng, Fuel, 2016, 171, 65–73 CrossRef CAS.
- H. Lim, L. Shagdarsuren, S. M. Kim, A. Hoshino, T. Yamashit and C. H. Jeon, J. Mech. Sci. Tech., 2016, 30, 1413–1420 CrossRef.
- P. Postorino, R. H. Tromp, M. A. Ricci and A. K. Soper, Nature, 1993, 366, 668–670 CrossRef CAS.
- Y. T. Xia, Y. K. Li, Y. T. Gu, T. Jin, Q. Yang, J. Hu, H. L. Liu and H. L. Wang, Fuel, 2016, 107, 100–106 CrossRef.
- X. J. He, N. Zhao, J. H. Qiu, N. Xiao, M. X. Yu, C. Yu, X. Y. Zhang and M. D. Zheng, J. Mater. Chem. A, 2013, 1, 9440–9448 CAS.
- W. C. Xu and A. Tomita, Fuel Process. Technol., 1989, 21, 25–37 CrossRef CAS.
- N. A. Oztas and Y. Yürüm, Fuel, 2000, 79, 1221–1227 CrossRef CAS.
- Y. Yürüm, R. Kramer and M. Levy, Thermochim. Acta, 1985, 94, 285–293 CrossRef.
- H. Mori, K. Asami and Y. Ohtsuka, Energy Fuels, 1996, 10, 1022–1027 CrossRef CAS.
- Y. Ohtsuka, T. Watanabe, K. Asami and H. Mori, Energy Fuels, 1998, 12, 1356–1362 CrossRef CAS.
- Y. Ohtsuka, C. B. Xu, D. P. Kong and N. Tsubouchi, Fuel, 2004, 83, 685–692 CrossRef CAS.
- X. Z. Gong, Z. C. Guo and Z. Wang, Energy Fuels, 2009, 23, 4547–4552 CrossRef CAS.
- Z. B. Ma, J. Bai, W. Li, Z. Q. Bai and L. X. Kong, Energy Fuels, 2013, 27, 4545–4554 CrossRef CAS.
- W. Li and Y. M. Zhu, Energy Fuels, 2014, 28, 3645–3654 CrossRef CAS.
- Y. D. Zhang, X. J. Kang and J. L. Tan, Energy Fuels, 2013, 27, 7191–7197 CrossRef CAS.
- X. J. Li, J. I. Hayashi and C. Z. Li, Fuel, 2006, 85, 1509–1517 CrossRef CAS.
- C. D. Sheng, Fuel, 2007, 86, 2316–2324 CrossRef CAS.
- A. Sasezky, H. Muckenhuber and H. Grothe, Carbon, 2005, 43, 1731–1742 CrossRef.
- T. T. Li, L. Zhang and D. Li, Fuel, 2014, 117, 1190–1195 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra14712g |
| This journal is © The Royal Society of Chemistry 2016 |






