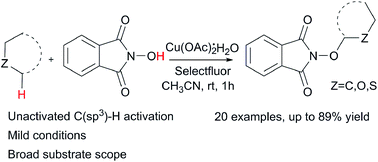
Copper(ii)-catalyzed cross dehydrogenative coupling reaction of N-hydroxyphthalimide with alkanes and ethers via unactivated C(sp3)–H activation at room temperature†
Abstract
A copper(II)-catalyzed cross dehydrogenative coupling reaction between N-hydroxyphthalimide and unactivated C(sp3)–H bonds of alkanes and ethers using Selectfluor as an oxidant is described. This efficient reaction system shows mild conditions and a broad substrate scope for the generation of O-substituted N-hydroxyphthalimide derivatives.

 Please wait while we load your content...
Please wait while we load your content...