Elegant cooperativity of noncovalent interactions in effective removal of Cu–EDTA from water via stepwise addition of polymer and surfactant
Abstract
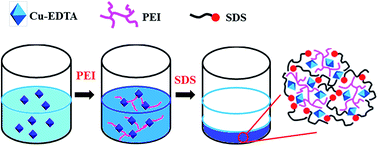
Removal of chelated metal ions from water remains a challenge in the treatment of industrial waste water since the chelating complexes are extremely stable, which means that precipitation is difficult. We report the elegant cooperativity of electrostatic interaction, coordinating interaction, and hydrophobic effects, which allows facile flocculation of Cu–EDTA through the stepwise addition of polyethyleneimine (PEI) and sodium dodecyl sulphate (SDS). The electrostatic interactions are important in ‘initiating’ the coordination between PEI and Cu–EDTA, and the hydrophobic interaction between SDS and PEI allows cross-linking of the PEI/Cu–EDTA complex to generate a precipitate. With this facile protocol, 97% of the Cu–EDTA complex in the waste water can be removed, and the residual level of Cu–EDTA can be lowered to below 2 mg L−1. Such cooperativity of noncovalent interactions is of great potential interest in the removal of chelated metal complexes from industrial water.

 Please wait while we load your content...
Please wait while we load your content...