Brönsted acid-mediated annulations of 1-cyanocyclopropane-1-carboxylates with arylhydrazines: efficient strategy for the synthesis of 1,3,5-trisubstituted pyrazoles†
Shuwen Xue,
Jiaming Liu,
Xushun Qing and
Cunde Wang*
School of Chemistry and Chemical Engineering, Yangzhou University, 180 Siwangting Street, Yangzhou 225002, P. R. China. E-mail: wangcd@yzu.edu.cn; Fax: +86-514-8797-5244; Tel: +86-514-8797-5568
First published on 12th July 2016
Abstract
1-Cyanocyclopropane-1-carboxylates are reacted with arylhydrazines to afford 1,3,5-trisubstituted pyrazoles under the influence of a Brönsted acid. Formally, this transformation can be regarded as an annulation of three-membered rings with α-carbonyl and hydrazines. This newly efficient method provides access to a variety of structurally diverse pyrazole derivatives. The structures of five typical products were confirmed by X-ray crystallography.
Cyclopropanes and their derivatives have been broadly used as valuable building blocks for the construction of various carbo- and heterocyclic systems due to their high π character, intrinsic ring strain and straightforward synthesis.1 They easily underwent a variety of ring-opening reactions, and the subsequent annulations with dipoles formed five-, six-, or seven-membered ring systems.2 Recently we reported the direct annulations of 2-aroyl-3-aryl-1-cyanocyclopropanecarboxylates with pyridine derivatives, nitromethane or β-nitrostyrenes to construct five-membered cyclic cores in a regioselective manner,3 which stimulated research into new pathways for the construction of cyclic units via the ring-opening reactions of 2-aroyl-3-aryl-1-cyanocyclopropanecarboxylates. Some previous studies revealed the most importantly synthetic value of donor–acceptor cyclopropanes has been extensively demonstrated in the preparation of highly substituted cyclic products via the vicinal arrangement of donor and acceptor moieties of cyclopropane that is able to stabilize a 1,3-zwitterionic relationship for formal [3 + 2] cycloaddition reactions.4
However, the main research efforts have been devoted to use the skeleton of cyclopropane for the construction of new cyclic skeletons via the ring-opening reactions of the common donor–acceptor cyclopropanes such as 2-arylcyclopropane 1,1-diesters. 2-Aroyl-3-aryl-1-cyanocyclopropanecarbonates could provide various 1,3-zwitterionic intermediate through different pathways for ring-opening due to involvement in conjugate hybrid from α-carbonyl group. Based on our studies on the reactivity of 2-aroyl-3-aryl-1-cyanocyclopropanecarbonates, we envisioned that the reaction of 2-aroyl-3-aryl-1-cyanocyclopropanecarbonates with arylhydrazines may offer an efficient approach to pyrazole skeletons. Although, the reported reactions of substituted cyclopropanes with hydrazines afforded pyridazin-3(2H)-ones and indole derivatives, or ring-opening products.2h,5 To the best of our knowledge, no example using the annulations of substituted cyclopropanes with arylhydrazines to afford 1,3,5-trisubstituted pyrazoles was reported. In this context, the annulation reactions of 1-cyanocyclopropane-1-carboxylates with arylhydrazines could provide an easy access to 1,3,5-trisubstituted pyrazoles under acidic conditions.
As is known to us, a pyrazole unit as a key structural motif exists in large numbers of pharmaceutically and agrochemically active compounds.6 Pyrazoles and their derivatives exhibit antimicrobial, antioxidant, antinflammatory, analgesic, anticonvulsant, anticancer, antiHIV-1 reverse transcriptase and herbicidal activities7 that are extensively used for the treatment of metabolic, CNS, and oncological diseases.8 For instances, a number of compounds containing a pyrazole scaffold have been successfully commercialized, such as Celebrex, Viagra, Fipronil, Lonazolac, Rimonabant, and Acomplia (Scheme 1).9 Additionally, pyrazole derivatives are also applied widely in supramolecular and polymer chemistry, in the food industry, and in the fine chemical industry such as fluorescent brightening agents, while some have liquid crystal properties.10
Pyrazole derivatives were usually synthesized through traditional approaches including the condensation of 1,3-dicarbonyl compounds with hydrazines and 1,3-dipolar cycloaddition of dipolarophiles with appropriate dipoles.11 The importance of the pyrazole moiety has prompted the development of many practical and efficient synthetic routes to construct their derivatives. Recently, the efficient methods have been developed with the aim of increasing the regioselectivity in the diverse synthesis of substituted pyrazoles. Among them, those involving the formation of C–N and C–C bonds by multicomponent reactions have emerged as a promising alternative to conventional methodologies.6b,10g,12 Nevertheless, the most common and efficiently synthetic method for the preparation of substituted pyrazoles still involves the cyclocondensation of an appropriate hydrazine, which acts as a double nucleophile, with a three-carbon unit featuring two electrophilic carbons in a 1,3-relationship, such as 1,3-dicarbonyl, α,β-unsaturated carbonyl compounds, and β-enaminones or related compounds. In addition, we have recently published a method for the generation of the specially 1,3-dipolar intermediate from 2-aroyl-3-aryl-1-cyanocyclopropanecarboxylates,3,13 which could allow for the construction of the pyrazole ring via annulation reaction with hydrazines.
At first, we treated the 1-cyanocyclopropane-1-carboxylate 1a with phenylhydrazine (2a, 2 equiv.) in AcOH as the solvent at 110 °C for 12 h (Table 1, entry 1). The desired product 3a was formed in 52% yield. The result indicated that acidic agents should promote the reaction. Subsequently, while inorganic acid H2SO4 was used as a promoter, we were pleased to find out that the yield was improved to 78% (Table 1, entry 2). Then, we examined the reaction using toluene as the solvent to replace AcOH at the above reaction. After overnight, the reaction yielded the required product in 82% yield (Table 1, entry 3). The preliminary results indicated the Brönsted acid and aprotic solvent could afford better results. Recently these studies showed that simple organic acids were used as green and highly efficient catalysts in the preparation of containing-nitrogen heterocycles.14 Thus, further many Brönsted acids such as sulfamic acid, malonic acid, benzoic acid, cinnamic acid, p-toluenesulfonic acid and inorganic acid phosphoric acid were screened (Table 1, entries 4–9), the desired product 3a was obtained in yield of 75%, 70%, 68%, 65%, 76%, and 81% respectively. Of these acids, sulfamic acid and p-toluenesulfonic acid gave the moderate yield (ca. 75%), however, the reaction needed the longer reaction time while p-toluenesulfonic acid was used. The use of malonic acid, benzoic acid, and cinnamic acid gave similar results (Table 1, entries 5–7). Under the same reaction conditions, phosphoric acid promoted the title reaction as much as the reaction activity of sulfuric acid (Table 1, entry 9), which indicated the detrimental effect of strong acids on the reaction. The results showed that sulphuric acid afforded the best result (84% in yield). Further study on the effect of temperature led to the observation that the reaction at 120 °C or 100 °C resulted in a lower yield of 80% and 78%, respectively (Table 1, entries 10–11). Further study on the effect of the amount of sulphuric acid led to the observation that the reaction using 0.8 equiv. H2SO4 afforded the best result (Table 1, entries 12 and 13). When the reaction was carried out in shorter reaction time (Table 1, entry 14) or in longer reaction time (Table 1, entry 15), the yield of product 3a was slightly decreased. Reducing the amount of phenylhydrazine (2a) to 1.5 equiv. did not afford the better result (Table 1, entry 17). Next, we studied the effect of solvent on reaction, the reaction was carried out with different solvents such as acetonitrile, 1,4-dioxane, xylene, cyclohexane and petroleum ether (90–120 °C) gave the desired product 3a in 65–82% of yields (Table 1, entries 17–21), screening of the solvents revealed that toluene and xylene were good candidates. Thus, reconsidering easy removal of toluene in the work-up step, we defined the reaction of the 1-cyanocyclopropane-1-carboxylate 1a with 2 equiv. phenylhydrazine (2a) and 0.8 equiv. sulphuric acid in toluene at 110 °C for 12 h as the standard conditions (Table 1, entry 10).
| Entry | Acid (equiv.)/solvent/T (°C)/ta (h) | Yieldb (%) |
|---|---|---|
| a Unless otherwise stated, reactions of 1a (1 mmol) and phenylhydrazine (2 mmol) were carried out in 5 mL of solvent.b Isolated yield.c 1a (1 mmol) and phenylhydrazine (1.5 mmol) were used.d PE: petroleum ether. | ||
| 1 | (−)/AcOH/110/12 | 52 |
| 2 | H2SO4 (1.0)/AcOH/110/12 | 78 |
| 3 | H2SO4 (1.0)/PhMe/110/12 | 82 |
| 4 | Sulfamic acid (1.0)/PhMe/110/12 | 75 |
| 5 | Malonic acid (1.0)/PhMe/110/12 | 70 |
| 6 | Benzoic acid (1.0)/PhMe/110/12 | 68 |
| 7 | Cinnamic acid (1.0)/PhMe/110/12 | 65 |
| 8 | p-TsOH (1.0)/PhMe/110/16 | 76 |
| 9 | H3PO4 (1.0)/PhMe/110/12 | 80 |
| 10 | H2SO4 (1.0)/PhMe/120/12 | 80 |
| 11 | H2SO4 (1.0)/PhMe/100/12 | 78 |
| 12 | H2SO4 (0.8)/PhMe/110/12 | 84 |
| 13 | H2SO4 (0.6)/PhMe/110/12 | 78 |
| 14 | H2SO4 (0.8)/PhMe/110/8 | 82 |
| 15 | H2SO4 (0.8)/PhMe/110/16 | 80 |
| 16c | H2SO4 (0.8)/PhMe/110/12 | 82 |
| 17 | H2SO4 (0.8)/CH3CN/reflux/12 | 70 |
| 18 | H2SO4 (0.8)/1,4-dioxane/110/12 | 65 |
| 19 | H2SO4 (0.8)/xylene/110/12 | 82 |
| 20 | H2SO4 (0.8)/cyclohexane/reflux/12 | 67 |
| 21d | H2SO4 (0.8)/PE (90–120 °C)/110/12 | 72 |
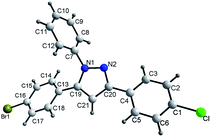
The structure of 3a was shown in Fig. 1.15 X-ray crystallographic analysis determined that product 3a possess three aryls contiguous substituents at N(1), C(3) and C(5). On the basis of spectroscopic evidence the structure of compound 3a was identified as 5-(4-bromophenyl)-3-(4-chlorophenyl)-1-phenyl-1H-pyrazole (3a).
The scope of this transformation was then investigated under the standard conditions using different 1-cyanocyclopropane-1-carboxylates, and substituted arylhydrazines. The results are summarized in Table 2. The reaction tolerated different substituents on the aromatic ring of the 1-cyanocyclopropane-1-carboxylates and arylhydrazines such as methyl, methoxy, chloro, iodo, and bromo at ortho-, meta- or para-positions of phenyl groups. Generally, substrates with para-position phenyl groups gave the products in higher yields than those with ortho-, or meta-position phenyl groups. The electronic properties of the substituents on the benzene ring of 1-cyanocyclopropane-1-carboxylates had a slight effect on the reaction. The introduction of an electron-withdrawing group such as Cl, Br or I speeded up the reaction and increased the yield of product, thus facilitating the synthesis of diversely substituted 1H-pyrazoles. Substrates with aryl and aroyl groups bearing electron-donating groups such as methyl, and methoxy afforded the corresponding pyrazoles in moderate yields (Table 2, entries 3, 5, 6, 9, 14, and 20). It is worth noting that substrates with aryl group bearing multi-substituents may also be applied (entries 10 and 12, Table 2). All corresponding substituted 1H-pyrazoles were analyzed by their 1H NMR, 13C NMR and MS. Characteristic 1H chemical shift of 1H-pyrazoles C(4)–H at δ ca. 6.75(s), unequivocally indicated the exclusive chemical environment of 1H-pyrazole C(4) proton. Products 1H-pyrazoles 3d, 3f, 3g and 3o were further characterized by single X-ray crystallography (Fig. 2).15
| Entry | R1 | R2 | R3 | 3 | Yieldb (%) |
|---|---|---|---|---|---|
| a 1-Cyanocyclopropane-1-carboxylates 1a–p (1 mmol), arylhydrazines 2a–c (2 mmol) and sulphuric acid (0.8 equiv.) toluene (5 mL), 110 °C, 12 h.b Isolated yield. | |||||
| 1 | p-Br | p-Cl | H | 3a | 84 |
| 2 | p-Cl | p-Cl | H | 3b | 87 |
| 3 | p-Cl | p-CH3O | H | 3c | 83 |
| 4 | p-Br | H | H | 3d | 82 |
| 5 | m-Cl | p-CH3O | H | 3e | 76 |
| 6 | o-Cl | p-CH3O | H | 3f | 78 |
| 7 | o-Cl | H | H | 3g | 79 |
| 8 | p-Br | p-CH3O | H | 3h | 80 |
| 9 | m-CH3 | p-CH3O | H | 3i | 72 |
| 10 | 3-F-4-PhO | p-Br | H | 3j | 81 |
| 11 | p-I | p-Br | H | 3k | 85 |
| 12 | 3-F-4-PhO | H | H | 3l | 80 |
| 13 | m-Cl | H | H | 3m | 75 |
| 14 | m-Cl | p-CH3O | p-Cl | 3n | 73 |
| 15 | p-Br | p-Cl | p-Cl | 3o | 87 |
| 16 | o-Cl | H | p-CH3 | 3p | 80 |
| 17 | p-CH3 | H | H | 3q | 81 |
| 18 | p-Br | p-CH3O | p-Cl | 3r | 84 |
| 19 | p-Br | p-Cl | p-CH3 | 3s | 85 |
| 20 | m-Br | p-CH3O | H | 3t | 73 |
On the basis of the above experimental results together with related reports, the reaction mechanism shown in Scheme 2 was proposed. In terms of pyrazole formations, the condensation of 1-cyanocyclopropane-1-carboxylates with arylhydrazine gave firstly the intermediate arylhydrazone [A], then donor–acceptor cyclopropane was attacked intramolecularly by α nitrogen of arylhydrazone, the following ring-opening reaction afforded dihydropyrazole salt [B].5 Next, the deprotonation of the dihydropyrazole salt [B] afforded dihydropyrazole [C]. Subsequent the elimination of ethyl cyanoacetate and aromatization furnished the pyrazole products 3a–t.
Conclusions
In conclusion, we have developed an efficiently Brönsted acid-promoted annulation reaction of 1-cyanocyclopropane-1-carboxylates with arylhydrazines to afford 1,3,5-trisubstituted pyrazoles in moderate to good yields (72–87%). This reaction involved the sequential condensation, intra-molecular addition/ring-opening reaction of 2-aroyl-3-aryl-1-cyanocyclopropanecarboxylates with arylhydrazines to give the corresponding pyrazoles. The development of this strategy offers a complementary approach to substituted pyrazole compounds with advantages that include a variety of cheap and readily available reactants and a wide range of substrates with dense or flexible substitution patterns.Acknowledgements
Financial support of this research by the National Natural Science Foundation of China (NNSFC 21173181) is gratefully acknowledged by authors. A Project was Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- (a) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151 CrossRef CAS PubMed; (b) F. Gnad and O. Reiser, Chem. Rev., 2003, 103, 1603 CrossRef CAS PubMed; (c) M. Yu and B. L. Pagenkopf, Tetrahedron, 2005, 61, 321 CrossRef CAS; (d) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117 CrossRef CAS PubMed; (e) D. Agrawal and V. K. Yadav, Chem. Commun., 2008, 44, 6471 RSC; (f) C. A. Carson and M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051 RSC; (g) F. De Simone and J. Waser, Synthesis, 2009, 3353 CAS; (h) D. Y. K. Chen, R. H. Pouwer and J. A. Richard, Chem. Soc. Rev., 2012, 41, 4631 RSC; (i) C. J. Thibodeaux, W. C. Chang and H. W. Liu, Chem. Rev., 2012, 112, 1681 CrossRef CAS PubMed; (j) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804 RSC; (k) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504 CrossRef CAS PubMed; (l) F. De Nanteuil, F. De Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Chem. Commun., 2014, 50, 10912 RSC; (m) S. H. Liao, X. L. Sun and Y. Tang, Acc. Chem. Res., 2014, 47, 2260 CrossRef CAS PubMed; (n) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655 RSC; (o) R. A. Novikov and Y. V. Tomilov, Mendeleev Commun., 2015, 25, 1 CrossRef CAS.
- (a) H. Xiong, H. Xu, S. Liao, Z. Xie and Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851 CrossRef CAS PubMed; (b) M. K. Ghorai, R. Talukdar and D. P. Tiwari, Chem. Commun., 2013, 49, 8205 RSC; (c) X. Ma, J. Zhang, Q. Tang, J. Ke, W. Zoub and H. Shao, Chem. Commun., 2014, 50, 3505 RSC; (d) H. K. Grover, M. R. Emmett and M. A. Kerr, Org. Biomol. Chem., 2015, 13, 655 RSC; (e) H. Liu, C. Yuan, Y. Wu, Y. Xiao and H. Guo, Org. Lett., 2015, 17, 4220 CrossRef CAS PubMed; (f) H. Xu, J. L. Hu, L. Wang, S. Liao and Y. Tang, J. Am. Chem. Soc., 2015, 137, 8006 CrossRef CAS PubMed; (g) R. A. Novikov, A. V. Tarasova, V. A. Korolev, E. V. Shulishov, V. P. Timofeev and Y. V. Tomilov, J. Org. Chem., 2015, 80, 8225 CrossRef CAS PubMed; (h) L. K. B. Garve, M. Petzold, P. G. Jones and D. B. Werz, Org. Lett., 2016, 18, 564 CrossRef CAS PubMed; (i) T. Yoshida, Y. Tajima, M. Kobayashi, K. Masutomi, K. Noguchi and K. Tanaka, Angew. Chem., Int. Ed., 2015, 54, 8241 CrossRef CAS PubMed; (j) L. K. B. Garve, M. Pawliczek, J. Wallbaum, P. G. Jones and D. B. Werz, Chem.–Eur. J., 2016, 22, 521 CrossRef CAS PubMed; (k) M. H. Shaw, N. G. McCreanor, W. G. Whittingham and J. F. Bower, J. Am. Chem. Soc., 2015, 137, 463 CrossRef CAS PubMed; (l) M. H. Shaw, R. A. Crofe, W. G. Whittingham and J. F. Bower, J. Am. Chem. Soc., 2015, 137, 8054 CrossRef CAS PubMed; (m) L. K. B. Garve, M. Pawliczek, J. Wallbaum, P. G. Jones and D. B. Werz, Chem.–Eur. J., 2016, 22, 521 CrossRef CAS PubMed.
- (a) J. Liu, L. Zhou, W. Ye and C. Wang, Chem. Commun., 2014, 50, 9068 RSC; (b) S. Xue, J. Liu and C. Wang, Eur. J. Org. Chem., 2016, 2450 CrossRef CAS; (c) C. Tan, W. Ye, J. Yao, J. Liu, S. Xue, Y. Li and C. Wang, RSC Adv., 2015, 5, 26491 RSC.
- M. S. Gordon, J. Am. Chem. Soc., 1980, 102, 7419 CrossRef CAS.
- (a) I. E. El-Kholy, H. M. Fuid-Alla and M. M. Mishrikey, J. Heterocycl. Chem., 1980, 17, 541 CrossRef; (b) D. Gladow and H.-U. Reissig, Helv. Chim. Acta, 2012, 95, 1818 CrossRef CAS; (c) S. S. So, T. J. Auvil, V. J. Garza and A. E. Mattson, Org. Lett., 2012, 14, 444 CrossRef CAS PubMed.
- (a) J. Elguero, in Comprehensive Heterocyclic Chemistry, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon, Oxford, 1996, vol. 5 Search PubMed; (b) S. Fustero, M. S. Rosell, P. Barrio and A. S. Fuentes, Chem. Rev., 2011, 111, 6984 CrossRef CAS PubMed; (c) A. Jamwal, A. Javed and V. J. Bhardwaj, J. Pharm. BioSci., 2013, 3, 114 Search PubMed; (d) P. A. Datar and S. R. Jadhav, Lett. Drug Des. Discovery, 2014, 11, 686 CrossRef CAS; (e) D. Pal, S. Saha and S. Singh, Int. J. Pharm. Pharm. Sci., 2012, 4, 98 CAS; (f) H. Wu, J. T. Feng, K. C. Lin and X. Zhang, Molecules, 2012, 17, 12187 CrossRef CAS PubMed; (g) A. Turetsky, E. Kim, R. H. Kohler, M. A. Miller and R. Weissleder, Sci. Rep., 2014, 4, 1 Search PubMed; (h) J. C. Byrd, et al., N. Engl. J. Med., 2013, 369, 32 CrossRef CAS PubMed; (i) A. Guzman-Perez, et al., Bioorg. Med. Chem. Lett., 2013, 23, 3051 CrossRef CAS PubMed; (j) S. Verstovsek, et al., N. Engl. J. Med., 2012, 366, 799 CrossRef CAS PubMed; (k) R. A. Mesa, U. Yasothan and P. Kirkpatrick, Nat. Rev. Drug Discovery, 2012, 11, 103 CrossRef CAS PubMed; (l) P. Koppikar, et al., Nature, 2012, 489, 155 CrossRef CAS PubMed; (m) Y. Xiong, et al., J. Med. Chem., 2012, 55, 6137 CrossRef CAS PubMed.
- (a) R. Nagamallu, B. Srinivasan, M. B. Ningappa and A. K. Kariyappa, Bioorg. Med. Chem. Lett., 2016, 26, 690 CrossRef CAS PubMed; (b) X. X. Tao, Y. T. Duan, L. W. Chen, D. J. Tang, M. R. Yang, P. F. Wang, C. Xu and H. L. Zhu, Bioorg. Med. Chem. Lett., 2016, 26, 677 CrossRef CAS PubMed; (c) J. Wen, Q. Niu, J. Liu, Y. Bao, J. Yang, S. Luan, Y. Fan, D. Liu and L. Zhao, Bioorg. Med. Chem. Lett., 2016, 26, 375 CrossRef CAS PubMed; (d) S. S. Abd El-Karim, M. M. Anwar, N. A. Mohamed, T. Nasr and S. A. Elseginy, Bioorg. Chem., 2015, 63, 1 CrossRef CAS PubMed; (e) X. H. Lv, Q. S. Li, Z. L. Ren, M. J. Chu, J. Sun, X. Zhang, M. Xing, H. L. Zhu and H. Q. Cao, Eur. J. Med. Chem., 2016, 108, 586 CrossRef CAS PubMed; (f) M. N. A. Nasr and S. A. Said, Arch. Pharm., 2003, 336, 551 CrossRef CAS PubMed; (g) Z. A. Kaplancikli, G. Turan-Zitouni, A. Ozdemir, G. Revial and K. Güven, Phosphorus, Sulfur Silicon Relat. Elem., 2007, 182, 749 CrossRef CAS; (h) N. Shankaraiah, V. Devaiah, K. L. Reddy, A. Juvekar, S. Sen, N. Kurian and S. Zingde, Bioorg. Med. Chem. Lett., 2008, 18, 1468 CrossRef PubMed.
- (a) T. Eicher, S. Hauptmann and A. Speicher, The Chemistry of Heterocycles, Wiley & Sons, 2nd edn, New York, 2004 Search PubMed; (b) N. R. Sperandeo and R. Brun, ChemBioChem, 2003, 4, 69 CrossRef CAS PubMed; (c) Z. Sui, J. Guan, M. P. Ferro, K. McCoy, M. P. Wachter, W. V. Murray, M. Singer, M. Steber, D. M. Ritchie and D. C. Argentieri, Bioorg. Med. Chem. Lett., 2000, 10, 601 CrossRef CAS PubMed; (d) A. A. Bekhit and T. Abdel-Aziem, Bioorg. Med. Chem., 2004, 12, 1935 CrossRef CAS PubMed; (e) C. Selvam, S. M. Jachak, R. Thilagavathi and A. K. Chakraborti, Bioorg. Med. Chem. Lett., 2005, 15, 1793 CrossRef CAS PubMed.
- (a) N. K. Terrett, A. S. Bell, D. Brown and P. Ellis, Bioorg. Med. Chem. Lett., 1996, 6, 1819 CrossRef; (b) T. D. Penning, J. J. Talley, S. R. Bertenshaw, J. S. Carter, P. W. Collins, S. Docter, M. J. Graneto, L. F. Lee, J. W. Malecha, J. M. Miyashiro, R. S. Rogers, D. J. Rogier, S. S. Yu, G. D. Anderson, E. G. Burton, J. N. Cogburn, S. A. Gregory, C. M. Koboldt, W. E. Perkins, K. Seibert, A. W. Veenhuizen, Y. Y. Zhang and P. C. Isakson, J. Med. Chem., 1997, 40, 1347 CrossRef CAS PubMed; (c) F. E. Chamberlain, C. Wang, H. Shi, D. C. Admas and Y. Ma, J. Agric. Food Chem., 2010, 58, 6895 CrossRef PubMed; (d) G. R. Bebernitz, G. Argentieri, B. Battle, C. Brennan, B. Balkan, B. F. Burkey, M. Eckhardt, J. Gao, P. Kapa, R. J. Strohschein, H. F. Schuster, M. Wilson and D. D. Xu, J. Med. Chem., 2001, 44, 2601 CrossRef CAS PubMed.
- (a) K. Dedelan, J. Shi, N. Shephered, E. Forsythe and D. C. Morton, Inorg. Chem., 2005, 44, 4445 CrossRef PubMed; (b) S. Y. Chang, J. L. Chen and Y. Chi, Inorg. Chem., 2007, 46, 11202 CrossRef CAS PubMed; (c) E. Cavero, S. Uriel, P. Romero, J. L. Serrano and R. Gimenez, J. Am. Chem. Soc., 2007, 129, 11608 CrossRef CAS PubMed; (d) C. Ye, G. L. Gard, R. W. Winter, R. G. Syvret, B. Twamley and J. M. Shreeve, Org. Lett., 2007, 9, 3841 CrossRef CAS PubMed; (e) L. Yang, F. Okuda, K. Kobayashi, K. Nozaki, Y. Tanabe, Y. Ishii and M. Haga, Inorg. Chem., 2008, 47, 7154 CrossRef CAS PubMed; (f) E. M. Chang, C. T. Lee, C. Y. Chen, F. F. Wong and M. Y. Yeh, Aust. J. Chem., 2008, 61, 342 CrossRef CAS; (g) L. Levi and T. J. J. Müller, Chem. Soc. Rev., 2016, 45, 2825 RSC.
- (a) A. A. Elkanzi, Int. J. Res. Pharm. Biomed. Sci., 2013, 4, 17 Search PubMed; (b) R. Aggarwal, V. Kumar, R. Kumar and S. P. Singh, Beilstein J. Org. Chem., 2011, 7, 179 CrossRef CAS PubMed; (c) S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111, 6984 CrossRef CAS PubMed; (d) R. E. Khidre, B. F. Abdel-Wahab, A. A. Farahat and H. A. Mohamed, J. Heterocycl. Chem., 2016, 53, 13 CrossRef CAS; (e) C. Guo, B. Sahoo, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2014, 136, 17402 CrossRef CAS PubMed; (f) V. Venkateswarlu, K. A. Aravinda Kumar, S. Balgotra, G. Lakshma Reddy, M. Srinivas, R. A. Vishwakarma and S. D. Sawant, Chem.–Eur. J., 2014, 20, 6641 CrossRef CAS PubMed; (g) P. A. Sokolyuk, I. S. Kondratov, O. V. Gavrylenko and A. A. Tolmachov, Mol. Diversity, 2016, 20, 1 CrossRef CAS PubMed; (h) L. Shi, L. Xue, R. Lang, C. Xia and F. Li, ChemCatChem, 2014, 6, 2560 CrossRef CAS; (i) M. Zora and A. Kivrak, J. Org. Chem., 2011, 76, 9379 CrossRef CAS PubMed; (j) Y. Schneider, J. Prévost, M. Gobin and C. Y. Legault, Org. Lett., 2014, 16, 596 CrossRef CAS PubMed; (k) A. A. Dissanayake and A. L. Odom, Chem. Commun., 2012, 48, 440 RSC.
- (a) B. Chen, C. Zhu, Y. Tang and S. Ma, Chem. Commun., 2014, 50, 7677 RSC; (b) P. Rai, M. Srivastava, J. Singh and J. Singh, RSC Adv., 2014, 4, 779 RSC; (c) M. Kashiwa, Y. Kuwata, M. Sonoda and S. Tanimori, Tetrahedron, 2016, 72, 304 CrossRef CAS; (d) M. A. Zolfigol, F. Afsharnadery, S. Baghery, S. Salehzadeh and F. Maleki, RSC Adv., 2015, 5, 75555 RSC; (e) Z. Li, J. Liu, Z. Huang, Y. Yang, C. Xia and F. Li, ACS Catal., 2013, 3, 839 CrossRef CAS.
- (a) W. Ye, L. Zhou, S. Xue, Y. Li and C. Wang, Synlett, 2015, 26, 1769 CrossRef CAS; (b) W. Ye, C. Tan, J. Yao, S. Xue, Y. Li and C. Wang, Adv. Synth. Catal., 2016, 358, 426 CrossRef CAS.
- (a) J. Aboonajmi, M. T. Maghsoodlou, N. Hazeri, M. Lashkari and M. Kangani, Res. Chem. Intermed., 2015, 41, 8057 CrossRef CAS; (b) Z. Madanifar, M. T. Maghsoodlou, M. Kangani and N. Hazeri, Res. Chem. Intermed., 2015, 41, 9863 CrossRef CAS; (c) M. Lashkari, M. T. Maghsoodlou, N. Hazeri, S. M. Habibi-Khorassani, S. S. Sajadikhah and R. D. Mohamadi, Synth. Commun., 2013, 143, 635 CrossRef; (d) S. S. Sajadikhah, M. T. Maghsoodlou, N. Hazeri, S. M. Habibi-Khorassani and S. J. Shamsnajafi, Monatsh. Chem., 2012, 143, 939 CrossRef CAS; (e) S. S. Sajadikhah, M. T. Maghsoodlou, N. Hazeri, S. M. Habibi-Khorassani and A. C. Willis, Chin. Chem. Lett., 2012, 23, 569 CrossRef CAS.
- Crystallographic data for 1H-pyrazoles 3a, 3d, 3f, 3g, 3o have been deposited with the Cambridge Crystallographic Data Centre with the deposition number CCDC 1427486, 1433734, 1465079, 1457746 and 1473251.
Footnote |
| † Electronic supplementary information (ESI) available: Reactions conditions and spectra. CCDC 1427486, 1433734, 1465079, 1457746 and 1473251. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra14557d |
| This journal is © The Royal Society of Chemistry 2016 |