Electrocatalytic performances of multi-walled carbon nanotubes chemically modified by metal phthalocyanines in Li/SOCl2 batteries
Weixing Yang,
Ronglan Zhang*,
Kai Luo,
Weiping Zhang and
Jianshe Zhao*
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry and Materials Science, Northwest University, Xi'an, Shaanxi 710069, China. E-mail: zhangrl@nwu.edu.cn; jszhao@nwu.edu.cn
First published on 26th July 2016
Abstract
A series of metal 2,9,16,23-tetraaminophthalocyanines [TAPcM, M = Cu(II), Ni(II), Zn(II), Fe(II), Mn(II)] have been prepared and used to chemically modify fluorinated multi-walled carbon nanotubes (F-MWCNTs). The generated composites TAPcM/MWCNTs were characterized by IR, UV-Vis and XRD. The electrocatalytic activity of all the composites was evaluated by adding them into the electrolyte of the Li/SOCl2 battery and taking cyclic voltammograms in simulated circumstances. The results show that the composites can dramatically improve the capacity of the battery, and their electrocatalytic performance strongly depends on the metal phthalocyanine species. The order of the electrocatalytic activity of 2,9,16,23-TAPcM and 2,9,16,23-TAPcM/MWCNTs with different metal ions in the center is Mn(II) > Ni(II) > Zn(II) > Fe(II) > Cu(II). The excellent electrocatalytic performance of the modified F-MWCNTs may be due to the fine synergistic effect between the metal phthalocyanines and the MWCNTs.
Introduction
Carbon nanotubes (CNTs), as unique one-dimensional nanostructures,1 have captured great research interest as a result of their high surface area, high thermal conductivity, excellent electrical conductivity and unique chemical properties.2–4 Importantly, the efforts to exploit the favorable properties of CNTs to apply them as electrocatalysts and electrode modifying materials is of fundamental interest.5–9 Due to their strong van der Waals attraction, CNTs tend to restack or aggregate both in a solution and in the solid state, which significantly decreases the active surface area, greatly hinders mass transport in the electrochemical process and often causes low reproducibility of their electrocatalytic properties. One of the effective strategies to overcome this demerit is surface modification of CNTs via covalent bonds.10,11 There are several advantages to this particular approach. First, the shape and size of the linker molecules can be easily tailored by sophisticated synthesis methods prior to combination with CNTs. Second, covalent bonds can rigidly connect the linker molecules and CNTs, such that the modified CNTs will not become easily dislodged even after sonication or extensive washing. Third, the spatial coverage and exact positions of the linker molecules depend on the precise nature of the chemical functionalities on the CNT surfaces, and these can be reasonably governed by controlling the oxidation treatments and chemical reaction conditions.11An Li/SOCl2 battery is a chemical power source, which has been applied in various fields due to its high specific energy, high voltage, long storage life and a wide range of operating temperature. The electrode reaction and the cell reaction of the Li/SOCl2 battery are shown in eqn (1)–(3).
Anode:
| 4Li − 4e → 4Li+ | (1) |
Cathode:
| 2SOCl2 + 4e → S↓ + SO2↑ + 4Cl− | (2) |
Total:
| 2SOCl2 + 4Li → S↓ + SO2↑ + 4LiCl↓ | (3) |
The cell reaction involves the formation of sulfur and insulating LiCl. The precipitation of LiCl and S is deposited on the electrode surface, which impede the progress of the discharge reaction. In order to conquer this demerit, numerous research studies have been conducted. Among these studies, metal phthalocyanine as an Li/SOCl2 battery electrocatalyst has spurred intense research interest and some progress has been achieved.12–14
Though metal phthalocyanines (MPcs) exhibit outstanding performance in the fields of electrocatalysts,12–16 their poor conductivity limits their wide utilization as favourable electrocatalysts. Recent research shows that CNT/MPc composites can bring a notable improvement in their electrocatalytic properties compared with the pristine CNTs or MPcs.17–22 In order to comprehensively utilize the advantages of carbon nanotubes and phthalocyanines, new material composites TAPcM/MWCNTs prepared and used as electrocatalysts for an Li/SOCl2 battery are presented in this study. The results indicate that the MWCNTs and metal phthalocyanines are linked covalently and the composites have an excellent electrocatalytic effect on the Li/SOCl2 battery. Their electrocatalytic activity is much higher than pristine metal tetraaminophthalocyanines and single F-MWCNTs.
Experimental
Materials and characterization
The reagents 4-nitrophthalic anhydride (89-40-7), ammonium molybdate (27546-07-2) and all metallic salts were obtained from J & K Chemical Technology Company. Urea (57-13-6) and Teflon emulsion (9002-84-0) were purchased commercially from Aladdin. Nitrobenzene (98-95-3), toluene (108-88-3), methanol (67-56-1), ether (60-29-7), ethyl acetate (141-78-6), hexane (110-54-3), pyridine (110-86-1), DMSO (67-68-5), and DMF (68-12-2) were of analytical grade and purchased from Tianjin Kemiou Chemical Reagent Co., Ltd [Tianjin, China]. F-MWCNTs (fluorinated multi-walled carbon nanotubes, F-3.06 wt%, 95%, length: 10–20 μm, CNT906) were purchased commercially from the Beijing Deke Daojin Science and Technology Co., Ltd [Beijing, China (Mainland)]. The LiAlCl4/SOCl2 electrolyte (battery grade), lithium pieces (battery grade), and carbon film (battery grade) were obtained from the Xi'an Institute of Electrical and Mechanical Services Information. IR spectra were obtained on a BRUKER VECTOR 22 spectrometer as KBr pellets. Absorption spectra were acquired on an American PE company, PE-40P UV spectrometer. Elemental analysis of the products was performed by a C, H, N analyzer model 1106 Carlo Erba Strumentazione. XRD patterns were obtained using a Bruker D8 Advance diffractometer equipped with Cu Kα radiation in 2θ range from 5° to 80°. Cyclic voltammetry was performed on a Chinese Zhengzhou Shiruisi Technology Co. Ltd RST5000 electrochemical workstation.Synthesis of 2,9,16,23-TAPcM and modification of F-MWCNTs
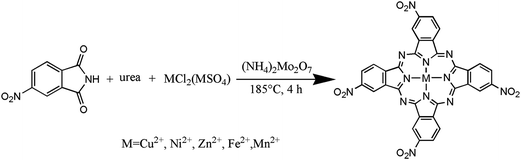
Synthesis of metal 2,9,16,23-tetranitrophthalocyanine [2,9,16,23-TNPcM, M = Cu(II), Ni(II), Zn(II), Fe(II), Mn(II)] was prepared following the route outlined in Scheme 1. Metal ions were used as a template to synthesize 2,9,16,23-TNPcM. Copper 2,9,16,23-tetranitrophthalocyanine (2,9,16,23-TNPcCu) was synthesized according to the literature.23 A 250 mL three-neck round-bottom flask was charged with nitrobenzene (30 mL). Then, 4-nitrophthalic anhydride (3.84 g, 0.02 mol), urea (6.00 g, 0.1 mol), and ammonium molybdate (0.10 g, 5.00 × 10−4 mol) were grinded equally. The grinded reactants were added to the flask and the mixture was stirred and refluxed for 30 min. When the solid chemicals were all dissolved, CuCl2·6H2O (1.00 g, 0.006 mol) was added to the flask. The mixture was heated to 185 °C and refluxed for 2 h, after that (2.00 g, 0.03 mol) urea was added to the reaction and the system continued the reflux for 2 h. When the reaction completed, the reacted system was cooled and diluted with toluene (80 mL). The resulting precipitate was filtered off using a G4 funnel under vacuum and sequentially washed with toluene, water, methanol/ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), and ethyl acetate/hexane (2
9), and ethyl acetate/hexane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). HCl (2%) and NaOH (2%) were used to wash the solid again until the filtrate was transparent, and finally, distilled water was used to wash the solid until it reached pH 7. The product was dried to obtain a blue solid of 2,9,16,23-TNPcCu 2.40 g, yield 64%, mp > 300 °C. Anal. calcd for C32H12N12O8Cu: C 50.83, H 1.60, N 22.23; found: C 50.66, H 1.61, N 22.18. IR (KBr pellet, cm−1): 1606 (w), 1522 (s), 1406 (w), 1335 (vs), 1255 (w), 1091 (s), 907 (s), 755 (s).
1). HCl (2%) and NaOH (2%) were used to wash the solid again until the filtrate was transparent, and finally, distilled water was used to wash the solid until it reached pH 7. The product was dried to obtain a blue solid of 2,9,16,23-TNPcCu 2.40 g, yield 64%, mp > 300 °C. Anal. calcd for C32H12N12O8Cu: C 50.83, H 1.60, N 22.23; found: C 50.66, H 1.61, N 22.18. IR (KBr pellet, cm−1): 1606 (w), 1522 (s), 1406 (w), 1335 (vs), 1255 (w), 1091 (s), 907 (s), 755 (s).
The general synthesis procedures of 2,9,16,23-TNPcM [M = Ni(II), Zn(II), Fe(II), Mn(II)] are the same as 2,9,16,23-TNPcCu.
Synthesis of metal 2,9,16,23-tetraaminophthalocyanine [2,9,16,23-TAPcM, M = Cu(II), Ni(II), Zn(II), Fe(II), Mn(II)] was prepared following the route outlined in Scheme 2. Na2S·9H2O (3.00 g, 0.0125 mol) was added to a DMF solution (30 mL) of 2,9,16,23-TNPcCu (0.76 g, 0.001 mol). The reaction mixture was heated to 60 °C and refluxed for 10 h. It was then cooled to room temperature. The cooled solution was diluted with ice water (100 mL), and the resulting precipitate was filtered off using a G4 funnel under vacuum. The precipitate was repeatedly washed with methanol/ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), ethyl acetate, carbon tetrachloride and ethanol, respectively, until the filtrate was transparent. It was dried to obtain a dark green solid copper 2,9,16,23-tetraaminophthalocyanine (2,9,16,23-TAPcCu) 0.61 g, yield 97%, mp > 300 °C. Anal. calcd for C32H20N12Cu: C 60.42, H 3.17, N 26.42; found: C 60.07, H 3.11, N 26.18. IR (KBr pellet, cm−1): 3335 (w), 3202 (w), 1609 (vs), 1409 (s), 1250 (s), 1093 (s), 908 (w), 758 (s).
9), ethyl acetate, carbon tetrachloride and ethanol, respectively, until the filtrate was transparent. It was dried to obtain a dark green solid copper 2,9,16,23-tetraaminophthalocyanine (2,9,16,23-TAPcCu) 0.61 g, yield 97%, mp > 300 °C. Anal. calcd for C32H20N12Cu: C 60.42, H 3.17, N 26.42; found: C 60.07, H 3.11, N 26.18. IR (KBr pellet, cm−1): 3335 (w), 3202 (w), 1609 (vs), 1409 (s), 1250 (s), 1093 (s), 908 (w), 758 (s).
 | ||
| Scheme 2 Synthesis of metal 2,9,16,23-tetraaminophthalocyanine [2,9,16,23-TAPcM, M = Cu(II), Ni(II), Zn(II), Fe(II), Mn(II)]. | ||
The general synthesis procedures of 2,9,16,23-TAPcM [M = Ni(II), Zn(II), Fe(II), Mn(II)] are the same as 2,9,16,23-TAPcCu.
Modification of F-MWCNTs
A mass of 0.25 g F-MWCNTs, which possesses 4.0 × 10−5 mol fluorine atoms, was added to a solution of 2,9,16,23-TAPcCu (0.33 g, 5.2 × 10−5 mol) in DMSO (50 mL) with a molar ratio of F and 2,9,16,23-TAPcCu of nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 in order to ensure that the F atoms in F-MWCNTs were fully modified. An aliquot of 0.25 mL pyridine as catalyst was also added.24 The resulting mixture was rapidly heated to 200 °C and refluxed for 24 h. The precipitate generated was filtered off using a G4 funnel under vacuum when it was still hot. In order to remove unreacted and adsorbed 2,9,16,23-TAPcCu on the surface of MWCNTs, the precipitate was repeatedly washed with DMSO, DMF, methanol and ethanol separately until the filtrate did not contain 2,9,16,23-TAPcCu (checked by the filtrate becoming colorless and transparent). The purified solid was dried to obtain black modified 2,9,16,23-TAPcCu/MWCNTs (0.51 g) with a greenish luster surface.
1.3 in order to ensure that the F atoms in F-MWCNTs were fully modified. An aliquot of 0.25 mL pyridine as catalyst was also added.24 The resulting mixture was rapidly heated to 200 °C and refluxed for 24 h. The precipitate generated was filtered off using a G4 funnel under vacuum when it was still hot. In order to remove unreacted and adsorbed 2,9,16,23-TAPcCu on the surface of MWCNTs, the precipitate was repeatedly washed with DMSO, DMF, methanol and ethanol separately until the filtrate did not contain 2,9,16,23-TAPcCu (checked by the filtrate becoming colorless and transparent). The purified solid was dried to obtain black modified 2,9,16,23-TAPcCu/MWCNTs (0.51 g) with a greenish luster surface.
The general modification procedure of F-MWCNTs with 2,9,16,23-TAPcM [M = Ni(II), Zn(II), Fe(II), Mn(II)] is the same as 2,9,16,23-TAPcCu. The as-prepared samples using 2,9,16,23-TAPcM [M = Ni(II), Zn(II), Fe(II), Mn(II)] are termed as 2,9,16,23-TAPcNi/MWCNTs (0.51 g), 2,9,16,23-TAPZn/MWCNTs (0.50 g), 2,9,16,23-TAPcFe/MWCNTs (0.49 g), and 2,9,16,23-TAPcMn/MWCNTs (0.47 g), respectively.
Electrochemical measurement and cyclic voltammetry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 wt%) in Teflon emulsion (60 wt%; it is diluted by a small amount of anhydrous ethanol) with an apparent area of 1 cm2, and a thickness of about 0.36 mm was used as the cathode.
7 wt%) in Teflon emulsion (60 wt%; it is diluted by a small amount of anhydrous ethanol) with an apparent area of 1 cm2, and a thickness of about 0.36 mm was used as the cathode.Results and discussion
IR spectra
The IR spectra were obtained for further investigation of the distinction among 2,9,16,23-TNPcM, 2,9,16,23-TAPcM and TAPcM/MWCNTs [M = Cu(II), Ni(II), Zn(II), Fe(II), Mn(II)] (Fig. 1).25 For 2,9,16,23-TNPcM (Fig. 1a), the strong absorption peaks of are in the range of 1512–1525 cm−1 (s) and 1320–1336 cm−1 (vs), respectively. Compared to 2,9,16,23-TNPcM, the two characteristic intense peaks of
are in the range of 1512–1525 cm−1 (s) and 1320–1336 cm−1 (vs), respectively. Compared to 2,9,16,23-TNPcM, the two characteristic intense peaks of 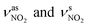 in 2,9,16,23-TAPcM (Fig. 1b) are absent. The new double peaks at 3300 cm−1 (s) and 3200 cm−1 (s), assigned to the stretching vibration of –NH2, appear in 2,9,16,23-TAPcM, which means that all the nitro-groups in 2,9,16,23-TNPcM compounds were transformed into amino-groups to produce 2,9,16,23-TAPcM compounds. For 2,9,16,23-TAPcM/MWCNTs (Fig. 1c), the single peaks of the stretching vibration of –NH are at about 3400 cm−1 (s), which indicates that –NH2 is changed to –NH. The IR data initially demonstrate that the 2,9,16,23-TAPcM compounds are bonding to F-MWCNTs through the imino bond (–NH).
in 2,9,16,23-TAPcM (Fig. 1b) are absent. The new double peaks at 3300 cm−1 (s) and 3200 cm−1 (s), assigned to the stretching vibration of –NH2, appear in 2,9,16,23-TAPcM, which means that all the nitro-groups in 2,9,16,23-TNPcM compounds were transformed into amino-groups to produce 2,9,16,23-TAPcM compounds. For 2,9,16,23-TAPcM/MWCNTs (Fig. 1c), the single peaks of the stretching vibration of –NH are at about 3400 cm−1 (s), which indicates that –NH2 is changed to –NH. The IR data initially demonstrate that the 2,9,16,23-TAPcM compounds are bonding to F-MWCNTs through the imino bond (–NH).
UV-vis spectra
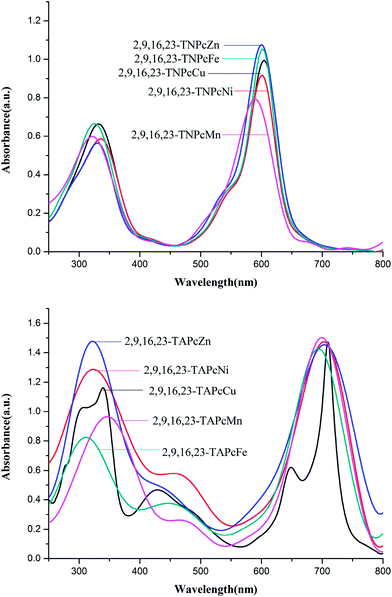
All compounds exhibited two absorption peaks corresponding to the characteristic B band (300–400 nm) and Q band (600–800 nm) of phthalocyanines (Fig. 2). These data support the evidence of microstructures of metal phthalocyanines.26 The two absorption peaks are caused by the π electron transition of the phthalocyanine ring.27 In 2,9,16,23-TAPcM compounds, weak absorption peaks at 400–500 nm result from the charge-transfer transition of amino groups to metals. Compared with the 2,9,16,23-TNPcM compounds, the electrophobic effect of amino groups gives rise to the red shift of the Q band in 2,9,16,23-TAPcM compounds.28 The data of the red shifts are shown in Table 1. All the Q bands of 2,9,16,23-TAPcM compounds are a red shift of about 100 nm (Table 1). There are almost no effects of B bands for these phthalocyanines.| 2,9,16,23-TAPc compound | Red shift of Q band (nm) |
|---|---|
| 2,9,16,23-TAPcCu(II) | 105 |
| 2,9,16,23-TAPcNi(II) | 102 |
| 2,9,16,23-TAPcZn(II) | 104 |
| 2,9,16,23-TAPcFe(II) | 92 |
| 2,9,16,23-TAPcMn(II) | 101 |
XRD analysis of the samples
XRD patterns of 2,9,16,23-TAPcM and 2,9,16,23-TAPcM/MWCNTs compounds are shown in Fig. 3. The XRD pattern of the pristine F-MWCNTs powder exhibits two characteristic diffraction peaks at 2θ = 26.542° (002) and 2θ = 44.517° (101) expected for hexagonal crystal system, space group P63/mmc phase, which is consistent with the standard card PDF #41-1487. This result manifests that F-MWCNTs possess a highly ordered structure. As for the patterns of 2,9,16,23-TAPcM compounds, two diffraction peaks appear in the range of 5–10° and some diffraction peaks appear in the range of 22–32°. The data are in agreement with that of copper phthalocyanine (PDF #36-1883), which indicates that the four amino substituents in the 2,9,16,23 positions of 2,9,16,23-TAPcM compounds do not affect the crystal structure (β-CuPc) of phthalocyanines. The XRD pattern of 2,9,16,23-TAPcM/MWCNTs presents both small angle diffraction peaks of 2,9,16,23-TAPcM compounds and the diffraction peaks (002, 101) of F-MWCNTs, which confirms that after 2,9,16,23-TAPcM compounds bond to F-MWCNTs, 2,9,16,23-TAPcM/MWCNTs still maintain a highly ordered structure. The highly maintained ordered structures of 2,9,16,23-TAPcM/MWCNTs provide for the rapid transmission of electrons, which is very significant for the electrocatalysts because electrons can move through them at an extremely high speed in the electrocatalytic process. The XRD results indicate that the modified F-MWCNTs should possess excellent electrocatalytic activity if the MWCNTs and metal phthalocyanines in them cooperate perfectly through the synergistic effect.17 This opinion is proven by the electrochemical assays for the modified F-MWCNTs, pristine F-MWCNTs and the corresponding tetraaminophthalocyanines.Electrocatalytic performance
As the reactions proceed (eqn (1)–(3)), the product SO2 dissolves in SOCl2 partially, and the insoluble LiCl membrane and S precipitate are precipitated out and deposited on the electrode surface.29 In addition, the battery anode lithium and SOCl2 also react according to the reaction: 8Li + 3SOCl2 → 6LiCl↓ + Li2SO3 + 2S↓. The products of the reactions can lead to harmful consequences. For example, voltage drop and low security. It is found that the fundamental cause of a voltage delay is the deposition of an LiCl film on the carbon cathode surface.30,31 To date, great advances have been achieved to suppress the voltage delay by adding chemical additives, such as metal phthalocyanines, porphyrins, and Schiff base complexes, to the battery solution32–35 to modify the passive film to increase its conductivity. Such additives do not, however, completely solve the passivation problem. Another excellent catalytic material bearing the performance of high speed for transmitting electrons should be discovered. Therefore, in this study, new material composites of TAPcM/MWCNTs were prepared and used as electrocatalysts for Li/SOCl2 batteries.In order to characterize the electrocatalytic performance of the composites of TNPcM/MWCNTs [M = Cu(II), Ni(II), Zn(II), Fe(II), Mn(II)] in the battery, each 2,9,16,23-TAPcM compound, composite and pristine F-MWCNTs were added to the electrolyte of the battery, and the relationship between the discharge voltage and the discharge time of the battery was recorded (Fig. 4). The bare represents the battery in the absence of catalyst.
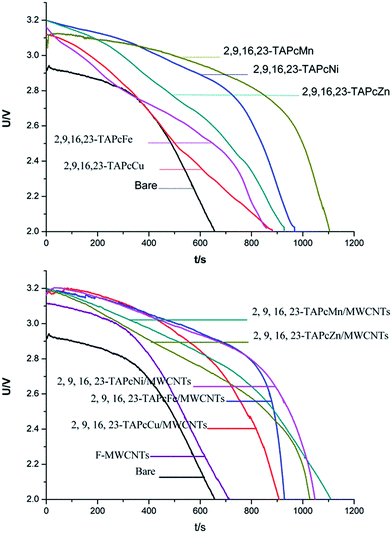 | ||
| Fig. 4 The U–t curves of the Li/SOCl2 battery (SOCl2 solution containing 2 mg 2,9,16,23-TAPcM compound and 2,9,16,23-TAPcM/MWCNTs composites, respectively). | ||
In order to quantify the electrocatalytic activity of the prepared electrocatalysts to the Li/SOCl2 battery and analyse the effect on the average voltage and capacity of the battery, numerical analyses were carried out (Table 2).
| Electrocatalyst | Umax (V) | Discharge time (s) | Vmax% | C (mA h) | C% |
|---|---|---|---|---|---|
| Bare | 2.943 | 657 | — | 12.1 | — |
| 2,9,16,23-TAPcCu(II) | 3.119 | 881 | 5.98 | 15.9 | 31.4 |
| 2,9,16,23-TAPcNi(II) | 3.199 | 968 | 8.69 | 19.3 | 59.5 |
| 2,9,16,23-TAPcZn(II) | 3.199 | 927 | 8.69 | 17.7 | 46.3 |
| 2,9,16,23-TAPcFe(II) | 3.159 | 858 | 7.33 | 16 | 32.2 |
| 2,9,16,23-TAPcMn(II) | 3.124 | 1105 | 6.15 | 22.1 | 82.6 |
| TAPcCu/MWCNTs | 3.194 | 907 | 8.53 | 18.2 | 50.4 |
| TAPcNi/MWCNTs | 3.204 | 1047 | 8.87 | 21.2 | 75.2 |
| TAPcZn/MWCNTs | 3.192 | 1027 | 8.46 | 19.9 | 64.5 |
| TAPcFe/MWCNTs | 3.194 | 928 | 8.53 | 19.2 | 58.7 |
| TAPcMn/MWCNTs | 3.201 | 1109 | 8.77 | 21.5 | 77.7 |
The capacity of the Li/SOCl2 battery is
 | (4) |
The rate capability (X) of the battery is
| X = (C − C0)/C0 × 100% | (5) |
In these formulae, C stands for the capacity of the battery in the presence of the catalysts, U stands for the output voltage of the battery, I stands for the discharge current of the battery, Δt stands for the discharge time of the battery, Re stands for the discharge resistance of the battery, and C0 stands for the capacity of the battery in the absence of the catalysts.
Along with the discharge time extension, the battery voltage reduces gradually (Fig. 4). Obviously, the discharge curves of the batteries in the presence of the catalysts are more stable than that of the battery in the absence of these catalysts (bare battery). In addition, the initial discharge voltage of the Li/SOCl2 battery catalyzed by the electrocatalysts increases approximately by 0.18–0.30 V and the discharge time lengthens by 224–452 s compared to that of the bare battery. These results indicate that the Li/SOCl2 battery containing 2,9,16,23-TAPcM compounds and their modified F-MWCNTs displays higher discharge voltage for a longer period of time. Therefore, the catalysts are beneficial for the battery to surmount the voltage delay problem and enhance the discharge performance. The capacity of Li/SOCl2 battery can also reflect the electrocatalytic activity of these catalysts (Table 2). The capacity of the battery in the presence of the catalysts is higher by 31.4–82.6% than that of the bare battery. All the results indicate these catalysts present relatively good catalytic performance to the Li/SOCl2 battery. All the 2,9,16,23-TAPcM compounds can improve the ceiling voltage of the batteries above 3.1 V, particularly 2,9,16,23-TAPcNi/MWCNTs, which can improve the ceiling voltage of the battery by 8.87%. For metal phthalocyanines containing different metal ions, it is found that 2,9,16,23-TAPcMn(II) and 2,9,16,23-TAPcNi(II) have a better effect on the stability of the battery voltage. They can increase the capacity of the batteries by 82.6% and 59.5%. This experimental fact demonstrates that the order of capacities of different central metals is as follows: Mn(II) > Ni(II) > Zn(II) > Fe(II) > Cu(II). Certainly, the electrocatalytic performance of the modified F-MWCNTs is better than their corresponding 2,9,16,23-TAPcM compounds (except 2,9,16,23-TAPcMn). They can improve the ceiling voltage of the battery by 8.77% (2,9,16,23-TAPMn/MWCNTs), 8.87% (2,9,16,23-TAPcNi/MWCNTs), 8.46% (2,9,16,23-TAPcZn/MWCNTs), 8.53% (2,9,16,23-TAPcFe/MWCNTs), 8.53% (2,9,16,23-TAPcCu/MWCNTs), and the capacity by 77.7% (2,9,16,23-TAPMn/MWCNTs), 75.2% (2,9,16,23-TAPcNi/MWCNTs), 64.5% (2,9,16,23-TAPcZn/MWCNTs), 58.7% (2,9,16,23-TAPcFe/MWCNTs), and 50.4% (2,9,16,23-TAPcCu/MWCNTs). The order of the electrocatalytic activities of the modified F-MWCNTs is also Mn(II) > Ni(II) > Zn(II) > Fe(II) > Cu(II). These results show that the electrocatalytic activities of catalysts with other identical motifs are different due to the diversity of central metal ions. This further indicates that the electrocatalytic activity of catalysts depends on not only the phthalocyanine rings and MWCNTs but also on the electronic configurations of the metal ions.13
By comparing the electrocatalytic activity of catalysts with the identical central metal ions, it is found that the electrocatalytic activity of Li/SOCl2 battery catalyzed by the modified F-MWCNTs is higher than that of the 2,9,16,23-TAPcM compounds (with the exception of 2,9,16,23-TAPcMn(II)). This is due to the fine synergistic effect between the metal phthalocyanines and the MWCNTs in them. Through the perfect cooperation of metal phthalocyanines and MWCNTs, the transportation of the electrons in the battery catalyzed by the modified F-MWCNTs is sped up sharply, which contributes to the major improvement of the capacity of the battery.17 In the manganese phthalocyanine system, the higher electrocatalytic activity of 2,9,16,23-TAPcMn(II) than its modified 2,9,16,23-TAPcMn/MWCNTs may be due to Mn2+ in 2,9,16,23-TAPcMn(II) with a d electronic configuration of 3d5 that is able to form an octahedral complex, which can form Mn3+ with a d electronic configuration of 3d4 via gaining one electron in the catalytic process. Mn3+ ion of 3d4 has the tendency to form a square planar complex and further enhance the process of the formation of the adduct 2,9,16,23-TAPcMn(II)·SOCl2 during the reduction of SOCl2, therefore enhancing the catalytic reaction.13
Cyclic voltammetric analysis
In order to intensively evaluate the catalytic performances of the modified F-MWCNTs to the Li/SOCl2 battery, CV tests were swept from 0 V to 5 V with 2 mg additives. The curve is recorded with a scanning rate of 0.1 V s−1 (Fig. 5). As seen from the linear cyclic voltammetry curve, there are no oxidation peaks appearing in the scanning process so the electron transfer coefficient cannot be calculated.36 However, two current peaks at 2.58 V and 3.57 V are found in the cathode curve of the bare, which are assigned to the reductions of SOCl2 and SCl2, respectively.17 That is, the cathode reaction (eqn (2)) of the Li/SOCl2 battery was divided into two steps (eqn (6) and (7)) in our simulated battery system.17 The high current peak at 2.58 V and the low current peak at 3.57 V mean that eqn (6) is the rate-controlling step and eqn (7) is the fast reaction step for the reductive reaction of SOCl2.33 Both peak potentials have migrations to the positive relative to the blank battery, as well as the peak currents. This evidence reveals that the entire catalytic process is not reversible37 and 2,9,16,23-TAPcM/MWCNTs systems come about in two-steps to transport electrons during the reduction processes.| SOCl2 + e → ½SCl2 + ½SO2 + Cl− | (6) |
| ½SCl2 + e → ½S + Cl− | (7) |
 | ||
| Fig. 5 Cyclic voltammograms of the Li/SOCl2 batteries (SOCl2 solution containing 2 mg 2,9,16,23-TAPcM–MWCNTs composites) at the scanning rate of 0.10 V s−1. | ||
From the perspective of numerical analyses, the maximum reduction peak potential of the most obvious catalyst (2,9,16,23-TAPcMn/F-MWCNTs) in the rate-controlling step (eqn (6)) for improving the battery performances has an elevated value of about 0.12 V, and for the lowest catalyst (2,9,16,23-TAPcCu/F-MWCNTs), it is 0.10 V. In the same way, the reduction peak potentials (3.58 V and 3.57 V) of SCl2 in the fast reaction step (eqn (7)) are unexpectedly found to be a little less than the blank experiment (3.57 V). However, the corresponding peak currents are 1.5 times and 1.2 times than that of the blank (0.00060 A), respectively. As a whole, the results of CV further manifest that phthalocyanine complexes that are modified by F-MWCNTs are conducive to the enhancement of catalytic activities for the Li/SOCl2 battery through the bonding effects. The introduction of an enormous surface area prompts TAPcM/F-MWCNTs systems to produce splendid electronic effects. Simultaneously, the p electrons from F-MWCNTs can develop large-scale delocalized π bonds giving rise to the strengthening of conjugated systems. Moreover, the CV findings are all in agreement with the results of the electrocatalytic performance for the modified F-MWCNTs.
Conclusions
In this investigation, a novel series of the metal phthalocyanines 2,9,16,23-tetraaminophthalocyanines [TAPcM, M = Cu(II), Ni(II), Zn(II), Fe(II), Mn(II)] were synthesized and modified successfully to F-MWCNTs. The electrocatalytic performance of compounds of 2,9,16,23-TAPcM and TAPcM/MWCNTs composites as an Li/SOCl2 battery was evaluated. The results indicate that these catalysts present relatively good catalytic performance as a Li/SOCl2 battery, particularly for the central ions containing Mn2+ and Ni2+. By CV analyses, the results demonstrated that the modified F-MWCNTs played a very important electrochemical role in improving the performance of the Li/SOCl2 battery. The experimental results show that the electrocatalysts possess excellent catalytic behavior through the perfect synergistic effect between the metal phthalocyanines and the MWCNTs.Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 21401149 and 21371143), the Science and Technology plan project of Xi'an City [No. CXY1438(6)], and the Natural Science Foundation of Shaanxi Province (No. 2015JM2062) for the financial support of this work.Notes and references
- A. Stojanovic, S. Olveira, M. Fischer and S. Seeger, Chem. Mater., 2013, 25, 2787–2792 CrossRef CAS.
- R. H. Baughman, A. A. Zakhidov and W. A. Heer, Science, 2002, 297, 787–792 CrossRef CAS PubMed.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- Y. P. Sun, K. Fu, Y. Lin and W. Huang, Acc. Chem. Res., 2002, 35, 1096–1104 CrossRef CAS PubMed.
- C. Han, A. Doepke, W. Cho, V. Likodimos, A. A. de la Cruz, T. Back and P. Falaras, Adv. Funct. Mater., 2013, 23, 1807–1816 CrossRef CAS.
- W. Grosse, J. Champavert, S. Gambhir, G. G. Wallace and S. E. Moulton, Carbon, 2013, 61, 467–475 CrossRef CAS.
- M. C. Gutiérrez, Z. Y. García-Carvajal, M. J. Hortigüela, L. Yuste, F. Rojo, M. L. Ferrer and F. del Monte, J. Mater. Chem., 2007, 17, 2992–2995 RSC.
- P. Li, H. Liu, J. Yang, D. Sun, Y. Chen, Y. Zhou and T. Lu, J. Mater. Chem. B, 2014, 2, 102–109 RSC.
- G. Lota, K. Fic and E. Frackowiak, Energy Environ. Sci., 2011, 4, 1592–1605 CAS.
- M. Y. Hua, Y. C. Lin, R. Y. Tsai and H. C. Chen, J. Mater. Chem., 2012, 22, 2566–2574 RSC.
- N. Karousis, N. Tagmatarchis and D. Tasis, Chem. Rev., 2010, 110, 5366–5397 CrossRef CAS PubMed.
- R. Zhang, J. Wang, B. Xu, X. Huang, Z. Xu and J. Zhao, J. Electrochem. Soc., 2012, 159, H704–H710 CrossRef CAS.
- Z. Xu, J. Zhao, H. Li, K. Li, Z. Cao and J. Lu, J. Power Sources, 2009, 194, 1081–1084 CrossRef CAS.
- W. Li, A. Yu, D. C. Higgins, B. G. Llanos and Z. Chen, J. Am. Chem. Soc., 2010, 132, 17056–17058 CrossRef CAS PubMed.
- T. Honda, T. Kojima and S. Fukuzumi, J. Am. Chem. Soc., 2012, 134, 4196–4206 CrossRef CAS PubMed.
- A. Sengül, H. Z. Dogan, A. Altındal, A. R. Ozkaya, B. Salih and O. Bekaroglu, Dalton Trans., 2012, 41, 7559–7572 RSC.
- R. Zhang, R. Wang, K. Luo, W. Zhang, J. Zhao and S. Zhang, J. Electrochem. Soc., 2014, 161, H941–H949 CrossRef CAS.
- G. Bottari, O. Trukhina, M. Ince and T. Torres, Coord. Chem. Rev., 2012, 256, 2453–2477 CrossRef CAS.
- R. Cao, R. Thapa, H. Kim, X. Xu, M. G. Kim, Q. Li and J. Cho, Nat. Commun., 2013, 4, 2076–2083 Search PubMed.
- A. Abbaspour and E. Mirahmadi, Electrochim. Acta, 2013, 105, 92–98 CrossRef CAS.
- X. Sun, F. Li, G. Shen, J. Huang and X. Wang, Analyst, 2014, 139, 299–308 RSC.
- I. M. Apetrei, M. L. Rodriguez-Mendez, C. Apetrei and J. A. De Saja, Sens. Actuators, B, 2013, 177, 138–144 CrossRef CAS.
- J. Alzeer, P. J. Roth and N. W. Luedtke, Chem. Commun., 2009, 15, 1970–1971 RSC.
- J. L. Stevens, A. Y. Huang, H. Peng, I. W. Chiang, V. N. Khabashesku and J. L. Margrave, Nano Lett., 2003, 3, 331–336 CrossRef CAS.
- J. Mack and M. J. Stillman, Coord. Chem. Rev., 2001, 219, 993–1032 CrossRef.
- M. Özil, E. Ağar, S. Şaşmaz, B. Kahveci, N. Akdemir and İ. E. Gümrükçüoğlu, Dyes Pigm., 2007, 75, 732–740 CrossRef.
- C. M. Allen, W. M. Sharman and J. E. Van Lier, J. Porphyrins Phthalocyanines, 2001, 5, 161–169 CrossRef CAS.
- J. A. Elvidge and A. B. P. Lever, J. Chem. Soc., 1961, 1257–1265 RSC.
- P. A. Bernstein and A. B. P. Lever, Inorg. Chem., 1990, 29, 608–616 CrossRef CAS.
- S. B. Lee, S. I. Pyun and E. J. Lee, Electrochim. Acta, 2001, 47, 855–864 CrossRef CAS.
- K. I. Chung, J. S. Lee and Y. O. Ko, J. Power Sources, 2005, 140, 376–380 CrossRef CAS.
- S. Szpak and J. R. Driscoll, J. Power Sources, 1983, 10, 343–354 CrossRef CAS.
- R. Zhang, B. Xu, J. Wang, J. Zhao and S. Zhang, J. Mater. Res., 2014, 29, 793–800 CrossRef CAS.
- X. Q. Su, J. Li, G. P. Yao, J. L. Wang, J. S. Zhao and F. X. Zhang, Catal. Commun., 2013, 37, 23–26 CrossRef CAS.
- W. S. Kim, W. J. Sim, K. I. Chung, Y. E. Sung and Y. K. Choi, J. Power Sources, 2002, 112, 76–84 CrossRef CAS.
- E. Laviron, J. Electroanal. Chem., 1979, 100, 263–270 CrossRef CAS.
- G. Shul, M. A. Murphy, G. D. Wilcox, F. Marken and M. Opallo, J. Solid State Electrochem., 2005, 9, 874–881 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |