CoOOH ultrafine nanoparticles for supercapacitors
Wei Wen*ab,
Dong Lianga,
Ji-Peng Chengbc and
Jin-Ming Wu*bc
aCollege of Mechanical and Electrical Engineering, Hainan University, Haikou 570228, P. R. China. E-mail: wwen@hainu.edu.cn
bState Key Laboratory of Silicon Materials, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: msewjm@zju.edu.cn
cSchool of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, P. R. China
First published on 22nd July 2016
Abstract
A novel synthetic strategy was developed to synthesize CoOOH ultrafine nanoparticles with a high surface area of 241 m2 g−1, which showed higher specific capacitance than other reported CoOOH powders when used as electrode materials of supercapacitors.
For decades, nanomaterials have sparked worldwide interest owing to their unique optical, electrical, magnetic and catalytic performances in comparison with those of bulk counterparts. Tuning the particle size is particularly an effective strategy to drastically enhance the physical/chemical properties of nanomaterials with increasing specific surface area.1,2 Ultrafine nanomaterials represent an exciting and challenging area of material synthesis and are promising candidates for many applications.3
Cobalt oxyhydroxide (CoOOH) is nonstoichiometric with a higher oxidation state Co (+3) than those in Co(OH)2 and Co3O4 and has a high electric conductivity of 5 S cm−1.4 CoOOH has attracted great research interest because of its potential applications in supercapacitors,4–14 electrocatalysis,15 photocatalysts,16 electrochemical sensors,17 fluorescence detection,18,19 gas sensors20 and alkaline Ni-metal hydride (MH) batteries.21 The preparation of CoOOH usually involves reactions under basic conditions in the presence of strong oxidizing reagents (for example S2O82−).22 Many nanostructures of CoOOH, such as nanorods,9,12,23 dumbbell-like structures,20 nanocrystals,24 nanorings,11 nanosheets4,13–19 and hollow spheres,25 have been synthesized; however, ultrafine CoOOH nanomaterials with a high specific surface area (above 100 m2 g−1) remain unexplored.
Herein, CoOOH ultrafine nanoparticles with a high specific surface area are fabricated by a novel synthetic route conducted at room temperature. The grain size is determined to be ca. 2.7 nm and the specific surface area is as high as 241 m2 g−1, with a mean pore size of ca. 6 nm. As an example of potential applications, the electrochemical properties of the CoOOH ultrafine nanoparticles as electrode materials of supercapacitors were demonstrated.
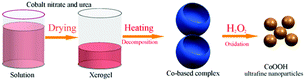
Fig. 1 schematically illustrates the synthetic procedure of CoOOH ultrafine nanoparticles. Inexpensive cobalt nitrate and urea were used as raw materials. Firstly, the decomposition/combustion reaction between cobalt nitrate and urea resulted in the formation of a blue Co-based amorphous complex. Then, the as-synthesized Co-based amorphous complex reacted with H2O2 to obtain the CoOOH ultrafine nanoparticles.
Metal nitrate and urea are widely used as oxidizer and reducer in solution combustion synthesis (SCS), respectively. In SCS, the stoichiometry of the fuel and oxidizer is usually calculated by propellant chemistry theory.26–28 The elemental valency of Co, C, H, N, and O is +2, +4, +1, 0, and −2, respectively. Accordingly, the reducing valency of urea and the oxidizing valency of cobalt nitrate are +6 and −10, respectively. The equilibrium combustion reaction between cobalt nitrate and urea can be described as,
 | (1) |
The cobalt-based complex was characterized by X-ray diffraction (XRD), field emission scanning electron microscope (FE-SEM), energy dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS), which are shown in Fig. 2. There is only a broad peak at ca. 23° in the XRD pattern (Fig. 2a), indicating the amorphous nature. The SEM (Fig. 2b) image shows that the amorphous cobalt-based complex has a hollow foam-like morphology. The cobalt-based complex contains Co, O, N and C elements, as shown in the EDX spectroscopy (Fig. 2c). The Al element is from the support for the sample during SEM tests. The mass fraction of C, N and H in the cobalt-based complex is 17.82%, 20.70% and 0.95%, measured by element analysis, respectively. The Co 2p spectrum in Fig. 2d shows four peaks of the 2p3/2 and 2p1/2 doublet. The Co 2p3/2 and 2p1/2 binding energies located at about 781.3 and 797.0 eV are in agreement with the binding energies of the photoelectrons of Co2+.25
 | ||
| Fig. 2 Characterizations of the cobalt-based complex: (a) XRD pattern, (b) SEM image, (c) EDX and (d) high-resolution Co 2p XPS spectrum. | ||
After reactions with H2O2, the cobalt-based complex converted to CoOOH phase with rhombohedral crystal system and a space group of R3m (JCPDS card no. 14-0673), as verified by XRD (Fig. 3a). Interestingly, the Bragg diffraction peaks are highly broadening, suggesting the ultrafine grains. The grain size is determined to be ca. 2.7 nm by Scherrer formula on the basis of full width at half maximum (FWHM) of the strongest peak. The mass fraction of N, C and H in the CoOOH is ca. 1%, 5% and 2%, as measured by the element analysis, which suggests that most of the organic components of the Co-based complex have been removed by the oxidation reactions and the Co-based complex has been converted to CoOOH. It is noted that the mass fraction of H in the CoOOH is higher than that in the Co-based complex, which is attributed to the larger amounts of adsorbed water in the CoOOH because of its high specific surface area (will be discussed later). A colloidal suspension of the CoOOH can be obtained by ultrasounding the CoOOH ultrafine nanoparticles in water. The suspension shows a brownish color and is stable over several days, displaying the Tyndall phenomenon that is characteristic of colloids, as shown in Fig. 3b. The microstructure of the CoOOH ultrafine nanoparticles was further analyzed by transmission electron microscope (TEM). Fig. 3c and d shows that the size of the CoOOH nanoparticles is typically less than 5 nm. These results indicate that the hollow foam-like morphology had transformed to ultrafine nanoparticles after the H2O2 treatment. It is found that the nanoparticles gather and grow together under electron beam irradiation during the TEM observations. The clear parallel lattice fringes in the high-resolution TEM (HRTEM) image (Fig. 3d) can be assigned to the interplanar spacing d101 (0.24 nm) of CoOOH. The SAED pattern (Fig. 3e) further confirms that the sample is CoOOH polycrystalline.
Fig. 3f shows the nitrogen adsorption–desorption isotherm and the pore-size distribution of the CoOOH ultrafine nanoparticles. It exhibits a type IV nitrogen isotherm with a type H3 desorption hysteresis loop at relative pressure range from 0.6 to 0.9, indicating the existence of mesopores. The specific surface area and total pore volume were determined to be 241 m2 g−1 and 0.58 cm3 g−1, respectively. On the contrary, the specific surface area of CoOOH nanomaterials reported in previous literature is typically less than 100 m2 g−1.4,9,10,22,29,30 To the best of our knowledge, it is the highest specific surface area for CoOOH previously reported.4,9,10,22,29,30 Furthermore, the pore-size distribution was obtained by the BJH approach from the desorption branch. The pore-size of CoOOH ultrafine nanoparticles mainly range from 5 to 15 nm, with a sharp peak at ca. 6 nm, as shown in the Inset of Fig. 3f. The high specific surface area of CoOOH ultrafine nanoparticles is attributed to its mesoporous structure and small particle size.
The valence of Co element in the amorphous cobalt-based complex is +2, which can be oxidized to +3 by H2O2 itself or O2 released from the decomposition of H2O2.25 It should be mentioned that the catalytic effect of cobalt-based complex results in the decomposition of H2O2 as below,
 | (2) |
Reaction (2) is a highly exothermic reaction, which results in the boiling of water. Thus, the temperature during the reaction between the cobalt-based complex and H2O2 was 100 °C, although the synthesis was conducted at room temperature. The increased temperature (100 °C) promotes the oxidation of Co2+ to Co3+ and removes the organic components of the cobalt-based complex, resulting in the formation of CoOOH. The initial hollow foam-like morphology was destroyed by the intensive reaction and the boiling of water. The high H2O2 concentration contributes to a high nucleation rate and the formation of lots of crystalline nuclei at the initial stage of reaction. The fast crystallization leads to a poor crystallinity of crystals. Moreover, the release of O2 and the boiling of water hinder the grain growth of CoOOH, resulting in the formation of ultrafine nanoparticles.
As an example of applications, the electrochemical properties of the CoOOH ultrafine nanoparticles as electrode materials of supercapacitors were investigated primarily. Fig. 4a shows the CV curves of the CoOOH ultrafine nanoparticles at the scan rates of 5–100 mV s−1. The shape of the CV curves is different from that of electric double layer capacitance (rectangular shape). The reduction and oxidation peaks in the CV curves suggest that the capacitance of CoOOH electrode is mainly from the faradaic redox reactions. The energy storage mechanism for CoOOH electrode in an alkaline electrolyte is as below,4
| CoOOH + OH− ↔ CoO2 + H2O + e− | (3) |
The specific capacitance (C) of CoOOH can be calculated from CV curves or charge–discharge curves according to the following eqn (4) or (5), respectively.
 | (4) |
| C = I × t/ΔU | (5) |
The specific capacitance (135 F g−1) of the CoOOH ultrafine nanoparticles at 1 A g−1 is higher than that of many other phase-pure CoOOH powders, such as porous hexagonal sheets (52 F g−1)13 and nanorings (ca. 100 F g−1);11 whilst it is equivalent to nanorods (136 F g−1)9,12 and CoOOH/CNTs (134 F g−1).10 The improved electrochemical performance of the CoOOH ultrafine nanoparticles can be attributed to their high specific surface area and large pore volume. The high specific surface area provides abundant interface for energy storage; whilst the mesoporous structure favors the ion diffusion. Although specific capacitance of the CoOOH ultrafine nanoparticles is lower than that of nanostructured CoOOH thin films,4–8 it is of challenge to maintain the excellent electrochemical performance for thin films at a high mass of active material loaded, because the specific capacitance rapidly decreases as mass loading of active materials increases. For example, Yang and co-workers31 showed that the specific capacitance of Ni/MnO2 nanocone arrays dramatically decreased from 632 F g−1 to 227 F g−1 at 5 mV s−1, when the mass loading of active materials increased from 0.05 mg cm−2 to 0.38 mg cm−2. Doping with other transition metal elements (such as Ni) or compositing with carbon materials (such as CNTs) is a very effective way to dramatically improve the specific capacitance of the CoOOH.10–12 Further work is still needed to improve the specific capacitance of the CoOOH ultrafine nanoparticles by doping or compositing.
Conclusions
In summary, CoOOH ultrafine nanoparticles were prepared by a novel two-step synthetic route: a cobalt-based complex was firstly synthesized by a simple and rapid pyrolysis of cobalt nitrate and urea; the reaction between the cobalt-based complex and H2O2 resulted in the formation of CoOOH ultrafine nanoparticles. The grain size is determined to be ca. 2.7 nm by the Scherrer formula. The specific surface area is as high as 241 m2 g−1, which is the highest specific surface area for CoOOH previously reported, to the best of our knowledge. When used as electrode materials of supercapacitor, the CoOOH ultrafine nanoparticles exhibited higher specific capacitance than other reported CoOOH powders because of their high specific surface area and large pore volume.Experimental section
Synthesis
In a typical procedure, 1.25 g of Co(NO3)2·6H2O and 2.1 g of urea (CH4N2O) were dissolved in 10 mL deionized water to form a clear solution. The solution was dried at 80 °C overnight and then transferred to a preheated furnace maintained at 400 °C for ca. 10 min to finish the decomposition/combustion reaction, which resulted in the blue foam of Co-based complex. The blue foams were then ground into powders. Finally, 200 mL H2O2 aqueous solution (30 wt%) was added to react with 0.2 g of the blue powders at ambient condition. Once the blue powders contacted with the H2O2 aqueous solution, the exothermic reactions resulted in the boiling of water; thus, the H2O2 aqueous solution was slowly added and then the solid products were collected by centrifugation. This process was repeated for seven times to thoroughly fulfill the transformation from the Co-based complex to CoOOH. The final products were washed by deionized water and ethanol for several times, followed by a drying at 80 °C.Characterizations
The XRD measurements were conducted on a XRD-6000 diffractometer (SHIMADZU) with a Cu Kα radiation, operated at 40 kV, 40 mA (λ = 0.15406 nm), with a scan speed of 1° min−1. The powder morphology was observed by FE-SEM (SU70) and TEM (FEI-F20, FEI, USA) working at 200 kV. The content of C, H and N elements was determined by elemental analysis (Flash EA 1112, ThermoFinnigan). The XPS characterization was carried out on an Escalab 250Xi system (Thermo Fisher Scientific). The binding energy (BE) was calibrated by using the containment carbon (C 1s = 284.6 eV). The specific surface area and pore diameter distributions were tested by N2 adsorption–desorption at 77 K (Quantachrome) using Brunauer–Emmett–Teller (BET) approach. The sample was degassed at 150 °C for 14 h to remove physisorbed gases prior to the measurement.Preparation of electrodes and electrochemical characterizations
The working electrodes were prepared by a slurry coating procedure. The slurry consisted of 80 wt% CoOOH powders, 10 wt% acetylene black and 10 wt% polyvinylidene fluoride (PVDF) dispersed in N-methyl pyrrolidinone (NMP), and was coated on a Ni foam substrate (1.5 × 1.5 cm2), which acted as a current collector. The electrodes were then dried at 80 °C in vacuum. The mass of the active material (CoOOH) loaded on each electrode was ca. 6.4 mg. The electrochemical measurements were performed in a standard three-electrode system in 1 M KOH aqueous solution. The Ni foam loaded with active material, Ag/AgCl electrode and Ni plate were used as working electrode, reference electrode and counter electrode, respectively. Cyclic voltammograms (CV) and galvanostatic charge–discharge curves were measured on a CHI660D electrochemical workstation (Chenhua, Shanghai). The cycle life test was conducted on a CT2001A test system (LAND, Wuhan) by galvanostatic charge–discharge techniques.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (No. 51502065), State Key Laboratory of Silicon Materials (No. SKL2016-12), and Hainan University (No. kyqd1541).References
- K. Yamamoto, T. Imaoka, W. J. Chun, O. Enoki, H. Katoh, M. Takenaga and A. Sonoi, Nat. Chem., 2009, 1, 397–402 CrossRef CAS PubMed.
- Y. D. Wang, I. Djerdj, M. Antonietti and B. Smarsly, Small, 2008, 4, 1656–1660 CrossRef CAS PubMed.
- S. Hu and X. Wang, Chem. Soc. Rev., 2013, 42, 5577–5594 RSC.
- E. Hosono, S. Fujihara, I. Honma, M. Ichihara and H. Zhou, J. Power Sources, 2006, 158, 779–783 CrossRef CAS.
- D. S. Dhawale, S. Kim, D. H. Park, J. H. Choy, S. S. Aldeyab, K. Ariga, E. Kim and A. Vinu, ChemElectroChem, 2015, 2, 497–502 CrossRef CAS.
- H. Zheng, F. Tang, M. Lim, A. Mukherji, X. Yan, L. Wang and G. Q. Lu, J. Power Sources, 2010, 195, 680–683 CrossRef CAS.
- H. Zheng, F. Tang, M. Lim, T. Rufford, A. Mukherji, L. Wang and G. Lu, J. Power Sources, 2009, 193, 930–934 CrossRef CAS.
- M. Wang, W. Ren, Y. Zhao and H. Cui, J. Nanopart. Res., 2014, 16, 2181 CrossRef.
- C. J. Raj, B. C. Kim, W. J. Cho, S. Park, H. T. Jeong, K. Yoo and K. H. Yu, J. Electroanal. Chem., 2015, 747, 130–135 CrossRef.
- M. Li, J. P. Cheng, F. Liu and X. B. Zhang, Electrochim. Acta, 2015, 178, 439–446 CrossRef CAS.
- Y. Chen, J. Zhou, P. Maguire, R. O' Connell, W. Schmitt, Y. Li, Z. Yan, Y. Zhang and H. Zhang, Sci. Rep., 2016, 6, 20704 CrossRef CAS PubMed.
- L. Zhu, W. Wu, X. Wang, X. Wu, W. Tang and Y. Wu, RSC Adv., 2014, 4, 59088–59093 RSC.
- M. S. Wu, C. Y. Tsai and Y. S. Lai, RSC Adv., 2015, 5, 15674–15681 RSC.
- A. D. Jagadale, D. P. Dubal and C. D. Lokhande, Mater. Res. Bull., 2012, 47, 672–676 CrossRef CAS.
- J. Huang, J. Chen, T. Yao, J. He, S. Jiang, Z. Sun, Q. Liu, W. Cheng, F. Hu, Y. Jiang, Z. Pan and S. Wei, Angew. Chem., Int. Ed., 2015, 127, 8846–8851 CrossRef.
- J. Huang, Q. Shang, Y. Huang, F. Tang, Q. Zhang, Q. Liu, S. Jiang, F. Hu, W. Liu, Y. Luo, T. Yao, Y. Jiang, Z. Pan, Z. Sun and S. Wei, Angew. Chem., Int. Ed., 2016, 55, 2137–2141 CrossRef CAS PubMed.
- K. K. Lee, P. Y. Loh, C. H. Sow and W. S. Chin, Biosens. Bioelectron., 2013, 39, 255–260 CrossRef CAS PubMed.
- Y. Cen, Y. Yang, R. Q. Yu, T. T. Chen and X. Chu, Nanoscale, 2016, 8, 8202–8209 RSC.
- G. Li, W. Kong, M. Zhao, S. Lu, P. Gong, G. Chen, L. Xia, H. Wang, J. You and Y. Wu, Biosens. Bioelectron., 2016, 79, 728–735 CrossRef CAS PubMed.
- B. Geng, F. Zhan, H. Jiang, Z. Xing and C. Fang, Cryst. Growth Des., 2008, 8, 3497–3500 CAS.
- W. Chen, Y. Yang and H. Shao, J. Power Sources, 2011, 196, 488–494 CrossRef CAS.
- C. H. Chen, S. F. Abbas, A. Morey, S. Sithambaram, L. P. Xu, H. F. Garces, W. A. Hines and S. L. Suib, Adv. Mater., 2008, 20, 1205–1209 CrossRef CAS.
- W. K. Hu, X. P. Gao, M. M. Geng, Z. X. Gong and D. Noreus, J. Phys. Chem. B, 2005, 109, 5392–5394 CrossRef CAS PubMed.
- S. R. Alvarado, Y. Guo, T. P. A. Ruberu, A. Bakac and J. Vela, J. Phys. Chem. C, 2012, 116, 10382–10389 CAS.
- J. Yang and T. Sasaki, Chem. Mater., 2008, 20, 2049–2056 CrossRef CAS.
- W. Wen, J. M. Wu and M. H. Cao, Nano Energy, 2013, 2, 1383–1390 CrossRef CAS.
- W. Wen, J. M. Wu and M. H. Cao, Nanoscale, 2014, 6, 12476–12481 RSC.
- W. Wen and J. M. Wu, RSC Adv., 2014, 4, 58090–58100 RSC.
- J. W. Wang and Y. M. Kuo, Phys. Status Solidi A, 2013, 210, 494–502 CrossRef CAS.
- L. B. Kong, M. Liu, J. W. Lang, Y. C. Luo and L. Kang, J. Electrochem. Soc., 2009, 156, A1000–A1004 CrossRef CAS.
- Z. Su, C. Yang, B. Xie, Z. Lin, Z. Zhang, J. Liu, B. Li, F. Kang and C. P. Wong, Energy Environ. Sci., 2014, 7, 2652–2659 CAS.
| This journal is © The Royal Society of Chemistry 2016 |