Photoresponsive nanostructured membranes†
Abstract
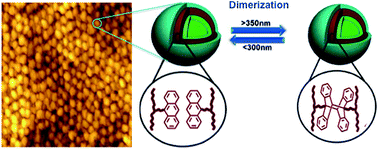
The perspective of adding stimuli-response to isoporous membranes stimulates the development of separation devices with pores, which would open or close under control of environment chemical composition, temperature or exposure to light. Changes in pH and temperature have been previously investigated. In this work, we demonstrate for the first time the preparation of photoresponsive isoporous membranes, applying self-assembly non-solvent induced phase separation to a new light responsive block copolymer. First, we optimized the membrane formation by using poly(styrene-b-anthracene methyl methacrylate-b-methylmethacrylate) (PS-b-PAnMMA-b-PMMA) copolymer, identifying the most suitable solvent, copolymer block length, and other parameters. The obtained final triblock copolymer membrane morphologies were characterized using atomic force and electron microscopy. The microscopic analysis reveals that the PS-b-PAnMMA-b-PMMA copolymer can form both lamellar and ordered hexagonal nanoporous structures on the membrane top layer in appropriate solvent compositions. The nanostructured membrane emits fluorescence due to the presence of the anthracene mid-block. On irradiation of light the PS-b-PAnMMA-b-PMMA copolymer membranes has an additional stimuli response. The anthracene group undergoes conformational changes by forming [4 + 4] cycloadducts and this alters the membrane's water flux and solute retention.

 Please wait while we load your content...
Please wait while we load your content...