Design and synthesis of a rhodol isomer and its derivatives with high selectivity and sensitivity for sensing Hg2+ and F− in aqueous media†
Jun Qin,
Huirong Yao‡
,
Song He and
Xianshun Zeng*
School of Materials Science & Engineering, Tianjin University of Technology, Tianjin 300384, China. E-mail: xshzeng@tjut.edu.cn; Fax: +86-22-60215226; Tel: +86-22-60216748
First published on 1st August 2016
Abstract
Because of their high extinction coefficients, high quantum yields, and reasonable water solubility, xanthene dyes have played crucial roles in the field of molecular imaging as fluorescent tracers. In this work, a novel hydroxyl regioisomeric rhodol 1 has been designed and synthesized. Both absorption and fluorescence data in water revealed that the rhodol isomer 1 can generate stable optical signals in the pH range 4–10. The maximum absorbance wavelength (ca. 540 nm) of 1 meets perfectly the discrete excitation of the laser at 539 nm, and the emission maximum wavelength (ca. 590 nm) is largely red shifted compared to that of the rhodol fluorophore (ca. 540 nm). Meanwhile, the dye could be easily designed to form chemodosimeters or probes by modification of the carboxyl group or the hydroxyl group. The hydrazide derivative and the silyl ether derivative of the dye in aqueous media exhibited excellent selectivity and sensitivity toward Hg2+ and F−, respectively. Thus, the excellent optical properties and chemical properties of this dye allow it to be designed as a fluorescent tracer for biological applications.
Introduction
The quest for fluorescent dyes is a continued attractive research theme with prime importance, as the signal detection via fluorescent methods relate to the fields of fluorescent sensing technology, fluorescent imaging technology, and photonic and electronic devices.1 It is well known that xanthene dyes, such as fluorescein and rhodamine derivatives, have several interesting innate photophysical natures, e.g. high extinction coefficients, high quantum yields, and reasonable biocompatibilities, which are highly expected for their use as signal reporters for fluorescence tracers.1,2 The merits for their use as fluorescent reporters in biological fields are that the intense absorption bands at ca. 490 nm for fluorescein derivatives and at ∼540 nm for rhodamine derivatives meet perfectly the discrete excitation of the laser at 488 nm and 539 nm, respectively, while also possessing reasonable quantum yields and excellent photostability in varieties of organic and aqueous media.3 To date, they have been widely used as fluorophore for the molecular design of chemosensors and probes.3,4 However, a few of intrinsic deficiencies limited their applications as fluorescent labels and tracers, such as fluorescein-based dyes showed pH-dependent fluorescence properties,4 irreversible photobleaching under the intense illumination,3,5 etc., and some rhodamine dyes exhibited strong tendency to form dimers.6,7As another member of xanthene family, rhodol fluorophore (Chart 1), a hybrid structure of fluorescein and rhodamine which also named “Rhodafluor”,5 has also been proved useful fluorophore for fluorescent sensors since it inherited all the photophysical properties from fluorescein and rhodamine, such as high extinction coefficients, quantum yields and photostability. It exhibited stable fluorescence emissions while the media pH is above 5.5. In the past few years, the rhodol fluorophore and its analogues have been successfully designed as a range of fluorescent probes, such as probes for all kinds of high reactive oxygen species,8 enzymes,9 and other chemical species.10–13 To the best of our knowledge, the rhodol fluorophore derivatives are quite limited to date. Inspired by the relationships between the structures and the photophysical and photochemical properties of this type of fluorophores, we assumed that the change of the position of the electron donor substituent of the dyes will alter the “push–pull” properties of its internal charge transfer (ICT) excited state of the parent fluorophore, which will allow us to optimize the photophysical properties of the dyes for the desired applications. Based on this idea, we herein reported the synthesis, photophysical properties and chemical properties of a 2′-hydroxyl-6′-(diethylamino)fluoran 1, an unique xanthene based hydroxyl regio-isomeric rhodol dye (Chart 1). The rhodol isomer 1 exhibited the following advantages as a potential fluorophore for the molecular design of fluorescent chemodosimeters and probes: (1) it showed reasonable water solubility because it kept a ring-opened zwitterion structure in aqueous phase; (2) compared with the maximum absorbance wavelength (ca. 520 nm) of rhodol fluorophore, the maximum absorbance wavelength (ca. 540 nm) of 1 meet perfectly the discrete excitation of the laser at 539 nm, and the emission maximum wavelength (ca. 590 nm) are largely red shifted than that of rhodol fluorophore (ca. 550 nm); (3) the rhodol isomer 1 showed strong pH tolerance, and it can generate stable optical signals in the pH range 4–10; (4) the rhodol isomer 1 can be easily chemical modified to produce all kinds of chemodosimeters and probes in two ways, e.g. via modification of the carboxyl group and the hydroxyl group. At the same time, a Hg2+-selective fluorescent chemodosimeter 2 was prepared via modification of the carboxyl group by hydrazine, and a F−-selective silyl ether 3 was synthesized via silylation of the hydroxyl group of this fluorescent dye (Chart 1). We anticipated that the excellent innate nature, such as water-soluble, pH-independent optical properties, and the easily chemical modification properties of the dye will allow it to be widely used as fluorophore for the molecular design of chemodosimeters and probes.
Results and discussion
Synthesis of the rhodol isomer 1
As shown in Scheme 1, the rhodol isomer 1 was facilely synthesized in one step by the reaction of 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid and hydroquinone at 90 °C for 24 h in methanesulfonic acid. After crystallized from ethyl acetate, the rhodol isomer 1 was obtained as a pink powder in 85% yield, which is pure enough for analyses and for the next reactions. The structure of the rhodol isomer 1 was confirmed by NMR and high-resolution mass spectra (HRMS).Modification of the rhodol isomer 1 as chemodosimeters
To get a general knowledge of its chemical properties, we then investigated the possibilities of the chemical modification of the rhodol isomer 1 as fluorescent chemodosimeters. It is well known that the hydrazide derivatives of xanthene dyes, such as rhodamine B and rhodamine 6G, showed excellent selectivity and sensitivity toward Cu2+ and Hg2+ cations,14,15 respectively. Thus, we firstly tried the modification of the carboxyl group by the reaction of 1 with hydrazine monohydrate. A Hg2+-selective hydrazide derivative 2 was obtained in this way. Meanwhile, silyl ether derivative of some fluorescent dyes have been reported to be F−-selective chemodosimeters or probes.16 Therefore, we then tried the modification of the hydroxyl group by the reaction of 1 with tert-butyldiphenylchlorosilane. A F−-selective silyl ether 3 was obtained under such a modification of this dye. Structures of the hydrazide derivative 2 and the silyl ether 3 were confirmed by NMR and HRMS spectra.Optical properties of the rhodol isomer 1
To understand the optical properties of the dye, we firstly explored the spectroscopic properties of the rhodol isomer 1 in solvents with different polarities in neutral and acidic media. As shown in Fig. 1a, the π–π* transitions of the ring-opened conjugate of 1 in water solutions showed two absorption bands at 506 nm (ε = 2.45 × 104 M−1 cm−1) and 540 nm (ε = 2.4 × 104 M−1 cm−1), respectively. It also showed a weak absorption at 546 nm (ε = 4 × 103 M−1 cm−1) in ethanol solutions. No absorption bands over 400 nm were observed in other organic solvents (Fig. 1a). Upon addition of TFA (TFA/solvent, 1%, v/v) in most-organic solvents, however, it immediately induced prominent absorbance enhancements at ca. 505 nm and ca. 540 nm, respectively (inset in Fig. 1a). A very weak increase of the absorbance bands over 400 nm in acidic dioxane was observed. It is interesting to note that there is no obvious affect on the absorption of 1 in neutral and acidic water solutions. The results revealed that the rhodol isomer 1 kept a ring-closed spirolactone structure in neutral organic solvents, but the spirolactone structure is very sensitive to acid in most organic solvents. However, it is in a ring-opened π-conjugate in both neutral and acidic water media. Then, the fluorescence emission properties of 1 were investigated. As shown in Fig. 1b, the rhodol isomer 1 in acidic solvents with different polarities showed maxima fluorescence emission bands between 586 nm and 598 nm, whereas the maximum fluorescence emission band in acidic THF was largely red-shifted to 624 nm. Compared with the rhodol fluorophore, the maximum absorption band of the rhodol isomer 1 is red-shifted 20 nm, and the maximum fluorescence emission band is red-shifted ca. 40 nm in water at pH 7 (Fig. S1a and b, ESI†).As described above, the rhodol isomer 1 kept in a ring-opened π-conjugate form in both neutral and acidic water media, which establishes the foundation to use this dye as a potential fluorophore for the molecular design of all kinds of fluorescent sensor. Thus, we next investigated the pH-dependent responses of the rhodol isomer 1 by absorption and fluorescence emission spectra in water solutions. 1 in water showed two strong absorption bands (at 505 nm and 540 nm, respectively) from pH 4 to 10.5 (Fig. S2a, ESI†). These two absorption bands are slightly red-shifted (ca. 5 nm) with the pH lower than 3, and they combined to a single band and red-shifted to 575 nm with the pH higher than 11. The pKa data of 1 derived from the pH-dependent absorption changes at 540 nm are 2.7 and 10.9, correspond to the interconversions between cationic and zwitterion forms, and the interconversions between zwitterion and anionic forms, respectively (Scheme 2). A pH-dependent fluorescent emission change of 1 in water was also investigated (Fig. S2b, ESI†). It showed a strong fluorescent emission band centered at ca. 585 nm from pH 4 to 10.5, and it slightly red-shifted to 590 nm with the pH lower than 3. However, the fluorescence is fully quenched with the pH higher than 11, indicated that the anionic form of 1 is nonfluorescence. The pKa derived from the pH-dependent fluorescence changes are 3.1 and 10.7, respectively. Both absorption and fluorescence data in water revealed that 1 kept a stable optical signals over a wide pH span from 4 to 10, which indicated that 1 established the foundation to be a pH-independent fluorophore for the molecular design of chemodosimeters.
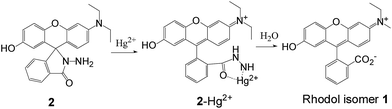
The molecular recognition properties of the hydrazide 2 toward Hg2+ ions
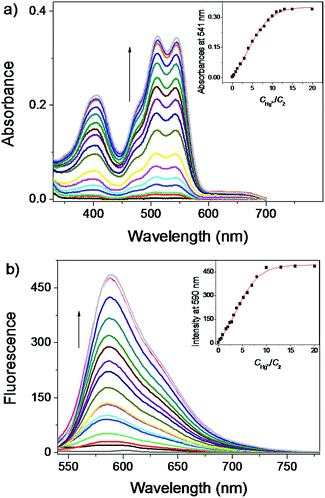
As described above, the excellent optical properties of the rhodol isomer 1 implied that, like classic rhodamines and fluorescein dyes, 1 could be employed as robust platforms for design of a range of fluorescent chemodosimeters. It has been well documented that many of the hydrazide derivatives of rhodamines had demonstrated excellent sensor properties towards Cu2+, Hg2+ or other chemical species.14,15 Thus, we prepared the hydrazide derivative 2 from the rhodol isomer 1 and investigated the molecular recognition properties of 2 towards a range of cations in water phase by UV-Vis absorption and fluorescence measurements.As shown in Fig. 2a and b, the hydrazide 2 showed no fluorescence and no absorption band over 400 nm in water, indicated that it kept a ring-closed circular spirolactam structure. However, addition of Hg2+ immediately elicited a dramatic change of the absorption bands over 400 nm and emission maximum at ca. 590 nm, which is similar to the spectroscopic features of the rhodol isomer 1. In addition, the obvious change of solution color in the presence of Hg2+ suggests that the hydrazide 2 would be a practical ‘naked-eye’ chemodosimeter of Hg2+ in water solutions (Fig. S3, ESI†). From the fluorescence titrations, the fluorescence turn-on constant (Kturn-on) was calculated to be (4 ± 0.088) × 10−5 M (with correlation coefficient R = 0.9976) (Fig. S4, ESI†).17 At the same time, the detection limit was estimated to be 3.31 × 10−9 M, which indicated that the limit of detection of the hydrazide 2 to Hg2+ met the limit for drinking water according to the China EPA standard (2 ppb, 10 nM) (Fig. S5, ESI†). Then, the response of the hydrazide 2 to other cations were investigated in water. There was no obvious effect on the UV-Vis absorption and fluorescence emission properties upon addition of 10 equivalents of a series of cations (Fig. S6a and b, ESI†). On the contrary, a prominent absorption enhancement over 400 nm and a significant enhancement of the emission intensity at 590 nm were observed upon addition of Hg2+. Further experiments for Hg2+-selective sensing were carried out with the hydrazide 2 in water with an excitation at 500 nm in the presence 10 equivalents of Ag+, Al3+, Ca2+, Cd2+, Co2+, Cr3+, Cu2+, Fe2+, Fe3+, K+, Mg2+, Na+, NH4+, Ni2+, Pb2+ and Zn2+. Upon addition of 10 equivalents of Hg2+, the fluorescent emission spectra and the emission intensities at 590 nm were almost identical to that obtained in the presence of Hg2+ alone (Fig. 3). Stoichiometry for 2 and Hg2+ was measured on the basis of the Job's plot and was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S7†). Meanwhile, fluorescence changes of 2 as a function of pH were recorded in the presence and absence of Hg2+ in H2O (Fig S8†). The chemodosimeter 2 exhibited very weak fluorescence emission over a pH range of 4–12. The fluorescence increased with the pH lower than 4 for the protonation of hydrazide moiety led to ring opening of spirolactam structure. Upon addition of Hg2+, the fluorescence exhibited prominent enhancement from pH 4 to 10. Thus, 2 was capable of detecting Hg2+ in the pH range 4–10. The results indicated that 2 can function as a highly selective and sensitive fluorescent probe for the Hg2+ cations in water.
1 (Fig. S7†). Meanwhile, fluorescence changes of 2 as a function of pH were recorded in the presence and absence of Hg2+ in H2O (Fig S8†). The chemodosimeter 2 exhibited very weak fluorescence emission over a pH range of 4–12. The fluorescence increased with the pH lower than 4 for the protonation of hydrazide moiety led to ring opening of spirolactam structure. Upon addition of Hg2+, the fluorescence exhibited prominent enhancement from pH 4 to 10. Thus, 2 was capable of detecting Hg2+ in the pH range 4–10. The results indicated that 2 can function as a highly selective and sensitive fluorescent probe for the Hg2+ cations in water.
To elucidate the binding mechanism of the chemodosimeter 2 with Hg2+, the 1H NMR titrations of 2 with Hg2+ were then carried out in DMSO-d6 (Fig. 4). As shown in Fig. 4a, the aromatic protons of 2 were well split except that the protons 4–6 are overlapped at 6.36 ppm. On addition 1.0 equiv. of the D2O Hg(ClO4)2 solutions, all the protons were slight low-field shifted (Fig. 4b). Meanwhile, the protons 4–6 were not only obviously low-field shifted but also well split at 6.49 ppm, 6.53 ppm and 6.75 ppm, respectively. Upon addition 2.0 equiv. of the Hg(ClO4)2, no obvious changes were observed for most of the protons except that the protons 4–6 were further low-field shifted (Fig. 4c). Further increase the Hg(ClO4)2 concentrations led to the 1H NMR spectra became complex for the formation of new species (Fig. 4d). High-resolution mass spectra (HRMS) indicated that the chemodosimeter 2 was hydrolyzed to rhodol isomer 1 by Hg2+ (Fig. S9†). Based on the results of 1H NMR titrations and HRMS, the recognition mechanism of the chemodosimeter 2 toward Hg2+ was proposed as Hg2+-promoted hydrolysis of the hydrazide moiety to the ring-opened rhodol isomer 1 as shown in Scheme 3.
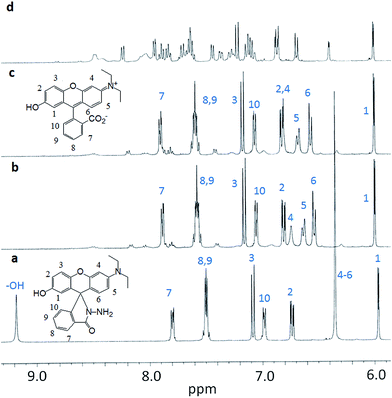 | ||
| Fig. 4 1H NMR titrations of 2 with Hg(ClO4)2 in DMSO-d6. (a) 2 in DMSO-d6; (b) 2 + 1 equiv. of Hg(ClO4)2; (c) 2 + 2 equiv. of Hg(ClO4)2; (d) 2 + 3 equiv. of Hg(ClO4)2. | ||
The recognition properties of the silyl ether 3 toward F− ions
It is well known that F− anions play a pivotal role in numerous health concerns, such as water depollution, metabolism disorders, dentistry.18 Whereas F− have many beneficial impacts on human health, larger amounts of F− exposure can lead to serious health problems, so that the World Health Organization recommends its content to be lower than 1.5 ppm in drinking water.19 However, a very large hydration enthalpy of F− makes the selective sensing of this anion in aqueous solutions a real challenge. In recent years, it has been reported that the alkyl/arylsilyl ether derivatives of some fluorophores showed excellent F− anion selectivities over other anions.16 Based on the excellent optical properties of the rhodol isomer 1, we prepared its tert-butyldiphenylsilyl ether derivative 3 to investigate F−-selective recognition behaviors in aqueous media.The fluorescence titration experiment was firstly conducted to evaluate the sensing property of the tert-butyldiphenylsilyl ether 3 to F− in DMSO–H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. Fig. 5 shows the spectroscopic changes of 3 at varied concentrations of NaF upon excitation at 514 nm. As expected, 3 displays a colorless solution and emits very weak fluorescence at ca. 590 nm in DMSO–H2O (v/v = 1
1) solution. Fig. 5 shows the spectroscopic changes of 3 at varied concentrations of NaF upon excitation at 514 nm. As expected, 3 displays a colorless solution and emits very weak fluorescence at ca. 590 nm in DMSO–H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution over a 12 h period, indicated that the Si–O bond has a strong resistance to be hydrolyzed by water. Addition of NaF, however, it produced a light red purple color with emission maximum at ca. 586 nm (Fig. 5). The changes in fluorescence titration spectra terminated when the concentration of F− reached 300 equivalents and over 10-fold fluorescence enhancement was reached under saturated conditions (ca. 300 equivalents). The fluorescence turn-on constant (Kturn-on) was calculated to be (9.1 ± 0.079) × 10−5 M (with correlation coefficient R = 0.9973) based on the fluorescence changes at 586 nm.17 In addition, a good linearity was obtained over the concentration range of 0–0.5 mM for F−. The regression equation was y = 71.22 + 0.76[F−] with a linear coefficient R of 0.998. From the F−-dependent fluorescence titrations (Fig. S10, ESI†), the detection limit was calculated to be 5.61 μM, indicating that the limit of detection of 3 to F− met the World Health Organization recommended threshold limit for fluoride content in drinking water (1.5 ppm).20
1) solution over a 12 h period, indicated that the Si–O bond has a strong resistance to be hydrolyzed by water. Addition of NaF, however, it produced a light red purple color with emission maximum at ca. 586 nm (Fig. 5). The changes in fluorescence titration spectra terminated when the concentration of F− reached 300 equivalents and over 10-fold fluorescence enhancement was reached under saturated conditions (ca. 300 equivalents). The fluorescence turn-on constant (Kturn-on) was calculated to be (9.1 ± 0.079) × 10−5 M (with correlation coefficient R = 0.9973) based on the fluorescence changes at 586 nm.17 In addition, a good linearity was obtained over the concentration range of 0–0.5 mM for F−. The regression equation was y = 71.22 + 0.76[F−] with a linear coefficient R of 0.998. From the F−-dependent fluorescence titrations (Fig. S10, ESI†), the detection limit was calculated to be 5.61 μM, indicating that the limit of detection of 3 to F− met the World Health Organization recommended threshold limit for fluoride content in drinking water (1.5 ppm).20
 | ||
Fig. 5 Fluorescence spectra of 3 (5 μM) in the presence of different concentrations of NaF in DMSO–H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. λex = 514 nm. Slit: 10 nm; 10 nm. 1) solution. λex = 514 nm. Slit: 10 nm; 10 nm. | ||
Subsequently, we investigated the response of the tert-butyldiphenylsilyl ether 3 (5.0 μM) to other anions in DMSO–H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. The addition of 40 equivalents of a series of anions, such as AcO−, Br−, Cl−, CO32−, H2PO4−, HCO3−, HPO42−, HSO4−, I−, NO2−, NO3−, PO43−, S2− and SO42−, has no prominent effect on the fluorescence emission properties (Fig. 6). However, an obvious enhancement (ca. 2-fold) of the emission intensity at 586 nm was observed upon addition of F−. Then, the selectivity of 3 to F− was further validated by competition experiments (Fig. 7). In the presence 40 equivalents of AcO−, Br−, Cl−, CO32−, H2PO4−, HCO3−, HPO42−, HSO4−, I−, NO2−, NO3−, PO43−, S2−, and SO42−, the addition of 40 equivalents of F− resulted almost identical fluorescence enhancements of 3 at 586 nm to that obtained in the presence of F− alone. Meanwhile, the fluorescence responses of 3 and 3 + F− from pH 4 to 12 were examined, which showed that 3 could be used within a wide pH span of 4.0–9.0 (Fig. S11, ESI†). As described above, the unique fluorescence emission changes of 3 with F− could be attributed to the cleavage of the Si–O bond by F−, which has been depicted in literatures (Fig. 8b).16 Free 3 is mainly in the non-fluorescent spirolactone structure in DMSO–H2O (v/v = 1
1) solution. The addition of 40 equivalents of a series of anions, such as AcO−, Br−, Cl−, CO32−, H2PO4−, HCO3−, HPO42−, HSO4−, I−, NO2−, NO3−, PO43−, S2− and SO42−, has no prominent effect on the fluorescence emission properties (Fig. 6). However, an obvious enhancement (ca. 2-fold) of the emission intensity at 586 nm was observed upon addition of F−. Then, the selectivity of 3 to F− was further validated by competition experiments (Fig. 7). In the presence 40 equivalents of AcO−, Br−, Cl−, CO32−, H2PO4−, HCO3−, HPO42−, HSO4−, I−, NO2−, NO3−, PO43−, S2−, and SO42−, the addition of 40 equivalents of F− resulted almost identical fluorescence enhancements of 3 at 586 nm to that obtained in the presence of F− alone. Meanwhile, the fluorescence responses of 3 and 3 + F− from pH 4 to 12 were examined, which showed that 3 could be used within a wide pH span of 4.0–9.0 (Fig. S11, ESI†). As described above, the unique fluorescence emission changes of 3 with F− could be attributed to the cleavage of the Si–O bond by F−, which has been depicted in literatures (Fig. 8b).16 Free 3 is mainly in the non-fluorescent spirolactone structure in DMSO–H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. With the Si–O bond breakage by F−, the non-fluorescent 3 was converted to the ring-opened fluorescent rhodol isomer 1 and led an unique fluorescence enhancement. Thus, 3 can function as a selective fluorescent chemodosimeter for F− anions via a F− promoted Si–O bond breakage within a wide pH span of 4.0–9.0.
1) solution. With the Si–O bond breakage by F−, the non-fluorescent 3 was converted to the ring-opened fluorescent rhodol isomer 1 and led an unique fluorescence enhancement. Thus, 3 can function as a selective fluorescent chemodosimeter for F− anions via a F− promoted Si–O bond breakage within a wide pH span of 4.0–9.0.
Then, the 1H NMR titrations of 3 with F− were investigated in details as shown in Fig. 8a. The numbering of protons of the rhodols skeleton for assignment of the 1H NMR spectra were illustrated in Fig. 8b. Before addition of NaF in DMSO-d6, the aromatic protons of 3 are well split except that the protons 4–6 are overlapped at 6.42–6.37 ppm. When 1.0 equiv. of NaF in D2O was added to the DMSO-d6 solution of 3, most of the aromatic protons were low-field shifted except that the protons 2 and 3 were high field-shifted. Further increase of the NaF concentrations (1.5–2.0 equiv.) led no obvious change of the 1H NMR spectra indicated the reaction was in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric. Meanwhile, there was no change can be observed from overnight.
1 stoichiometric. Meanwhile, there was no change can be observed from overnight.
Conclusion
In summary, a novel water-soluble and pH-independent fluorescent dye, the rhodol isomer 1 has been developed successfully from commercially available hydroquinone and 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid. Both absorption and fluorescence data in water revealed that 1 could generate stable optical signals in the pH range 4–10. Compared with those of rhodol fluorophore, the maximum absorption (ca. 540 nm) and fluorescent emission (ca. 585 nm) of 1 are obviously red-shifted. Meanwhile, the dye could be easily designed to chemodosimeters in two ways. A Hg2+-selective hydrazide derivative 2 was obtained via modification of the carboxyl group by the reaction of 1 with hydrazine monohydrate. At the same time, a F−-selective silyl ether 3 was obtained via silylation of the hydroxyl group by the reaction of 1 with tert-butyldiphenylchlorosilane. The molecular recognition properties of 2 and 3 were investigated in detail. 2 and 3 showed high selectivity and sensitivity toward Hg2+ and F−, respectively. An ongoing theme in our laboratory is the exploration of the dye as tracers in applications requiring pH tolerance.Experimental
General methods
All solvents and reagents (analytical grade and spectroscopic grade) were obtained commercially and used as received unless otherwise mentioned. NMR spectra were recorded on a Varian Mercury Vx-300 at 300 (1H NMR), Bruker spectrometer at 400 (1H NMR) MHz and 100 (13C NMR) MHz. Chemical shifts (δ values) were reported in ppm down field from internal Me4Si (1H and 13C NMR). High-resolution mass spectra (HRMS) were acquired on an Agilent 6510 Q-TOF LC/MS instrument (Agilent Technologies, Palo Alto, CA) equipped with an electrospray ionization (ESI) source. Elemental analyses were performed on a Vanio-EL elemental analyzer (Analysensystem GmbH, Germany). UV absorption spectra were recorded on a UV-2550 UV-VIS spectrophotometer (Shimadzu, Japan). Fluorescence measurements were performed using an F-4600 fluorescence spectrophotometer (Hitachi, Japan) equipped with a quartz cell (1 cm × 1 cm). Melting points were recorded on a RY-2 Melting Point Analyzer (Analytical Instrument Factory, Tianjin) and are uncorrected.Synthesis of 2′-hydroxyl-6′-(diethylamino)fluoran 1 (rhodol isomer 1)
To a 25 mL flask, was charged 2-(4-diethylamino-2-hydroxybenzoyl)benzoic acid (1.57 g, 5 mmol), hydroquinone (0.88 g, 8 mmol), methanesulfonic acid (5 mL). The reaction mixture was stirred at 90 °C for 24 h. After being cooled to room temperature, the reaction mixture was poured into ice water (50 mL). The pH of the mixture was adjusted to ∼7 by the addition of NaHCO3. The mixture was extracted with ethyl acetate (50 mL × 3). The combined organic layers were dried over anhydrous sodium sulfate. After filtration, the filtrate was condensed to dryness to afford the crude product. The crude product was recrystallized from ethyl acetate to afforded pure 1 as pink powder in 85% yield; mp: 188–190 °C. HRMS: m/z [M + H]+ = 388.1549; calcd: 388.1471; 1H NMR (400 MHz, DMSO-d6, ppm): 9.32 (s, 1H), 8.02 (d, J = 8 Hz, 1H), 7.81 (t, J = 8 Hz, 1H), 7.73 (t, J = 8 Hz, 1H), 7.30 (d, J = 8 Hz, 1H), 7.20 (d, J = 8 Hz, 1H), 6.89 (q, J = 4 Hz, 1H), 6.50–6.42 (m, 3H), 6.06 (d, J = 4 Hz, 1H), 3.34 (t, J = 8 Hz, 7H), 1.09 (t, J = 8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6, ppm): 169.2, 153.5, 153.0, 149.7, 144.6, 136.0, 129.1, 126.7, 125.0, 124.6, 119.1, 116.1, 112.4, 108.9, 104.3, 97.3, 84.2, 44.2, 12.8.Synthesis of the hydrazide 2
To a 25 mL flask, was charged 1 (3 g, 7.8 mmol), hydrazine (5 mL), ethanol (30 mL). The mixture was heated to reflux for 24 h under nitrogen atmosphere. After being cooled down to room temperature, the solvent was filtered. The filtrate was evaporated to dryness. The residue was recrystallized from methanol to afforded pure 2 as white powder in 61% yield; mp: 220–222 °C. HRMS: m/z [M + H]+ = 402.1819; calcd: 402.1739; 1H NMR (400 MHz, DMSO-d6, ppm): 8.76 (s, 1H), 7.59 (d, J = 8 Hz, 1H), 7.43 (t, J = 8 Hz, 1H), 7.24 (t, J = 8 Hz, 1H), 7.18 (d, J = 8 Hz, 1H), 7.09 (d, J = 8 Hz, 1H), 6.67 (q, J = 8 Hz, 1H), 6.44 (t, J = 8 Hz, 2H), 6.32 (d, J = 8 Hz, 1H), 6.28 (d, J = 8 Hz, 1H), 3.76–3.49 (m, 2H), 3.36 (q, J = 8 Hz, 4H), 1.19 (t, J = 8 Hz, 6H); 13C NMR (100 MHz, DMSO-d6, ppm): 167.00, 153.61, 153.26, 151.67, 145.86, 133.01, 128.55, 128.27, 127.61, 123.78, 122.88, 118.46, 118.04, 111.42, 77.36, 77.04, 76.73, 66.82, 44.42, 12.56.Synthesis of the tert-butyldiphenylsilyl ether 3
To a 50 mL flask, was added the rhodol isomer 1 (387.4 mg, 1 mmol), tert-butyldiphenylchlorosilane (549.7 mg, 2 mmol), anhydrous dichloromethane (10 mL). To this solution, triethyl-amine (303.6 mg, 3 mmol) was added dropwise. After the addition of triethylamine, the reaction mixture was stirred overnight at room temperature. Then, the reaction mixture was poured in water (50 mL). The organic layer was separated, and dried over sodium sulfate. After filtration, the filtrate was condensed to dryness. The residue was purified by crystallization from dichloromethane to afford 3 (417 mg) as a white powder in 67% yield; mp: 84–86 °C. HRMS: m/z [M + H]+ = 626.2784; calcd: 626.2727. 1H NMR (400 MHz, DMSO-d6, ppm): 0.92 (s, 9H), 1.04 (t, J = 7.0 Hz, 6H), 3.30 (q, J = 6.8 Hz, 4H), 5.83 (d, J = 2.8 Hz, 1H), 6.37 (q, J = 9.6 Hz, 3H), 6.97–7.03 (m, 2H), 7.21 (d, J = 8.8 Hz, 1H), 7.28–7.35 (m, 6H), 7.38–7.45 (m, 4H), 7.58–7.64 (m, 2H), 7.78–7.80 (m, 1H); 13C NMR (100 MHz, DMSO-d6, ppm): δ 12.7, 19.2, 26.8, 44.2, 83.7, 97.2, 104.1, 107.0, 117.1, 118.5, 119.9, 123.1, 123.9, 125.0, 126.3, 126.4, 126.9, 130.3, 130.6, 130.7, 131.8, 132.1, 135.2, 135.8, 146.0, 149.8, 150.9, 151.8, 152.9, 168.7.Acknowledgements
We gratefully acknowledge the Natural Science Foundation of China (NNSFC 21272172; 11432016), and the Natural Science Foundation of Tianjin (12JCZDJC21000).Notes and references
- (a) F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376 CrossRef PubMed; (b) J. J. McEwen and K. J. Wallace, Chem. Commun., 2009, 6339 RSC; (c) F. Würthner, Chem. Commun., 2004, 1564 RSC; (d) Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC; (e) R. S. Lokey and B. L. Iverson, Nature, 1995, 375, 303 CrossRef CAS; (f) S. Grimme, J. Antony, T. Schwabe and C. Mück-Lichtenfeld, Org. Biomol. Chem., 2007, 5, 741 RSC; (g) K. Y. Law, Chem. Rev., 1993, 93, 449 CrossRef CAS; (h) A. R. A. Palmans and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8948 CrossRef CAS PubMed.
- (a) K. C. Hannah and B. A. Armitage, Acc. Chem. Res., 2004, 37, 845 CrossRef CAS PubMed; (b) K. E. Achyuthan, T. S. Bergstedt, L. Chen, R. M. Jones, S. Kumaraswamy, S. A. Kushon, K. D. Ley, L. Lu, D. McBranch, H. Mukundan, F. Rininsland, X. Shi, W. Xia and D. G. Whitten, J. Mater. Chem., 2005, 15, 2648 RSC; (c) D. G. Whitten, K. E. Achyuthan, G. P. Lopez and O.-K. Kim, Pure Appl. Chem., 2006, 78, 2313 CrossRef CAS; (d) O.-K. Kim, J. Je, G. Jernigan, L. Buckley and D. Whitten, J. Am. Chem. Soc., 2006, 128, 510 CrossRef CAS PubMed; (e) R. F. Pasternack, A. Giannetto, P. Pagano and E. Gibbs, J. Am. Chem. Soc., 1991, 113, 7799 CrossRef CAS; (f) Y. Zhang, J. Xiang, Y. Tang, G. Xu and W. Yan, ChemPhysChem, 2007, 8, 224 CrossRef CAS PubMed; (g) Q. Yang, J.-F. Xiang, S. Yang, Q. Li, Q. Zhou, A. Guan, L. Li, Y. Zhang, X. Zhang, H. Zhang, Y. Tang and G. Xu, Anal. Chem., 2010, 82, 9135 CrossRef CAS PubMed; (h) J. Chang, Y. Lu, S. He, C. Liu, L. Zhao and X. Zeng, Chem. Commun., 2013, 49, 6259 RSC.
- (a) T. Tani, Photographic Sensitivity, Oxford University Press, Oxford, UK, 1995 Search PubMed; (b) D. Möbius, Adv. Mater., 1995, 7, 437 CrossRef.
- (a) G. McDermott, S. M. Prince, A. A. Freer, A. M. Hawthornthwaite-Lawless, M. Z. Papiz, R. J. Cogdell and N. W. Isaacs, Nature, 1995, 374, 517 CrossRef CAS; (b) T. Pullerits and V. Sundström, Acc. Chem. Res., 1996, 29, 381 CrossRef CAS; (c) Y. Nakamura, N. Aratani and A. Osuka, Chem. Soc. Rev., 2007, 36, 831 RSC; (d) S. Ganapathy, G. T. Oostergetel, P. K. Wawrzyniak, M. Reus, A. G. M. Chew, F. Buda, E. J. Boekema, D. A. Bryant, A. R. Holzwarth and H. J. M. de Groot, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 8525 CrossRef CAS PubMed.
- (a) G. Scheibe, Kolloid-Z., 1938, 82, 1 CrossRef CAS; (b) B. Kopainsky, J. K. Hallermeier and W. Kaiser, Chem. Phys. Lett., 1981, 83, 498 CrossRef CAS; (c) F. Würthner, Synthesis, 1999, 2103 CrossRef.
- (a) Y. Huang and J. M. Coull, J. Am. Chem. Soc., 2008, 130, 3238 CrossRef CAS PubMed; (b) W. Zhan, H. N. Barnhill, K. Sivakumar, H. Tiana and Q. Wang, Tetrahedron Lett., 2005, 46, 1691 CrossRef CAS; (c) Z. Q. Guo, W. Q. Chen and X. M. Duan, Org. Lett., 2010, 12, 2202 CrossRef CAS PubMed; (d) L. Yuan, W. Lin and J. Song, Chem. Commun., 2010, 46, 7930 RSC.
- (a) T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687 CrossRef CAS PubMed; (b) A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973 CrossRef CAS PubMed.
- (a) M. Hentschel, W. Dreher, P. Wust, S. Röll, D. Leibfritz and R. Felix, Phys. Med. Biol., 1999, 44, 2397 CrossRef CAS PubMed; (b) J. Olsrud, R. Wire-stam, S. Brockstedt, A. M. K. Nilsson, K.-G. Tranberg, F. Ståhlberg and B. R. R. Persson, Phys. Med. Biol., 1998, 43, 2597 CrossRef CAS PubMed; (c) S. Uchiyama, A. P. de Silva and K. Iwai, J. Chem. Educ., 2006, 83, 720 CrossRef CAS; (d) W. West and S. Pearce, J. Phys. Chem., 1965, 69, 1894 CrossRef CAS; (e) H. Ecker, Kolloid-Z., 1940, 92, 35 CrossRef CAS; (f) C. H. Fan and J. P. Longtin, J. Heat Transfer, 2000, 122, 757 CrossRef CAS; (g) J. F. Horton and J. E. Peterson, J. Heat Transfer, 2000, 122, 165 CrossRef CAS.
- (a) S. W. Allison and G. T. Gillies, Rev. Sci. Instrum., 1997, 68, 2615 CrossRef CAS; (b) J. P. Byrne, J. Chem. Educ., 1978, 55, 267 CrossRef CAS.
- (a) F. Ye, C. Wu, Y. Jin, Y.-H. Chan, X. Zhang and D. T. Chiu, J. Am. Chem. Soc., 2011, 133, 8146 CrossRef CAS PubMed; (b) C. Li and S. Liu, Chem. Commun., 2012, 48, 3262 RSC; (c) C. A. Naini, S. Franzka, S. Frost, M. Ulbricht and N. Hartmann, Angew. Chem., Int. Ed., 2011, 50, 4513 CrossRef CAS PubMed; (d) F. Robert, A. D. Naik, B. Tinant, R. Robiette and Y. Garcia, Chem.–Eur. J., 2009, 15, 4327 CrossRef CAS PubMed; (e) J. Harada, T. Fujiwara and K. Ogawa, J. Am. Chem. Soc., 2007, 129, 16216 CrossRef CAS PubMed; (f) S. Uchiyama, N. Kawai, A. P. de Silva and K. Iwai, J. Am. Chem. Soc., 2004, 126, 3032 CrossRef CAS PubMed; (g) C. Pietsch, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 5653 CrossRef CAS PubMed; (h) J. Feng, K. Tian, D. Hu, S. Wang, S. Li, Y. Zeng, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2011, 50, 8072 CrossRef CAS PubMed; (i) L. Yin, C. He, C. Huang, W. Zhu, X. Wang, Y. Xu and X. Qian, Chem. Commun., 2012, 48, 4486 RSC; (j) C. Pietsch, U. S. Schubert and R. Hoogenboom, Chem. Commun., 2011, 47, 8750 RSC; (k) A. R. Sheth, J. W. Lubach, E. J. Munson, F. X. Muller and D. J. W. Grant, J. Am. Chem. Soc., 2005, 127, 6641 CrossRef CAS PubMed.
- (a) J.-R. Chen and D.-Y. Yang, Org. Lett., 2009, 11, 1769 CrossRef CAS PubMed; (b) J.-J. Chen, K.-T. Li and D.-Y. Yang, Org. Lett., 2011, 13, 1658 CrossRef CAS PubMed; (c) J. R. Koe, M. Motonaga, M. Fujiki and R. West, Macromolecules, 2001, 34, 706 CrossRef CAS; (d) E. Yashima, K. Maeda and O. Sato, J. Am. Chem. Soc., 2001, 123, 8159 CrossRef CAS PubMed; (e) K. Yoshino, S. Nakajima and R. Sigimoto, Jpn. J. Appl. Phys., 1987, 26, L1038 CrossRef CAS.
- (a) M. Mitsuishi, S. Kikuchi, T. Miyashita and Y. Amao, J. Mater. Chem., 2003, 13, 2875 RSC; (b) D. H. Nash and I. R. Dempster, Eng. Failure Anal., 2005, 12, 699 CrossRef; (c) M. E. Köse, B. F. Carroll and K. S. Schanze, Langmuir, 2005, 21, 9121 CrossRef PubMed; (d) G. E. Khalil, C. Costin, J. Crafton, G. Jones, S. Grenoble, M. Gouterman, J. B. Callis and L. R. Dalton, Sens. Actuators, B, 2004, 97, 13 CrossRef CAS; (e) J. P. Hubner, B. F. Carroll, K. S. Schanse, H. F. Ji and M. S. Holden, AIAA J., 2001, 39, 654 CrossRef.
- (a) K. Binnemans, Chem. Rev., 2009, 109, 4283 CrossRef CAS PubMed; (b) C. Po, A. Y.-Y. Tam, K. M.-C. Wong and V. W.-W. Yam, J. Am. Chem. Soc., 2011, 133, 12136 CrossRef CAS PubMed; (c) S. Perruchas, N. Desboeufs, S. Maron, X. F. Le Goff, A. Fargues, A. Garcia, T. Gacoin and J.-P. Boilot, Inorg. Chem., 2012, 51, 794 CrossRef CAS PubMed; (d) A. A. Rachford and F. N. Castellano, Inorg. Chem., 2009, 48, 10865 CrossRef CAS PubMed; (e) L. H. Fischer, M. I. J. Stich, O. S. Wolfbeis, N. Tian, E. Holder and M. Schäferling, Chem.–Eur. J., 2009, 15, 10857 CrossRef CAS PubMed.
- (a) Y. Liu, Y. Sun, J. Du, X. Lv, Y. Zhao, M. Chen, P. Wang and W. Guo, Org. Biomol. Chem., 2011, 9, 432 RSC; (b) V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386 CrossRef CAS; (c) J. F. Zhang, Y. Zhou, J. Yoon, Y. Kim, S. J. Kim and J. S. Kim, Org. Lett., 2010, 12, 3852 CrossRef CAS PubMed.
- (a) N. R. Chereddy, P. Nagaraju, M. V. N. Raju, K. Saranraj, S. Thennarasu and V. J. Rao, Dyes Pigm., 2015, 112, 201 CrossRef CAS; (b) M. Wang, J. Wen, Z. Qin and H. Wang, Dyes Pigm., 2015, 120, 208 CrossRef CAS; (c) V. K. Bhardwaj, H. Sharma, N. Kaur and N. Singh, New J. Chem., 2013, 37, 4192 RSC; (d) C. Arivazhagan, R. Borthakur and S. Ghosh, Organometallics, 2015, 34, 1147 CrossRef CAS.
- (a) R. Hu, J. Feng, D. Hu, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2010, 49, 4915 CrossRef CAS PubMed; (b) S. Y. Kim, J. Park, M. Koh, S. B. Park and J.-I. Hong, Chem. Commun., 2009, 4735 RSC; (c) B. Zhu, F. Yuan, R. Li, Y. Li, Q. Wei, Z. Ma, B. Du and X. Zhang, Chem. Commun., 2011, 47, 7098 RSC.
- P. Du and S. J. Lippard, Inorg. Chem., 2010, 49, 10753 CrossRef CAS PubMed.
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192 CrossRef CAS PubMed; X. Li, X. Gao, W. Shi and H. Ma, Chem. Rev., 2014, 114, 590 CrossRef PubMed.
- (a) S. Ayoob and A. K. Gubta, Crit. Rev. Environ. Sci. Technol., 2006, 36, 433 CrossRef CAS; (b) P. Singh, M. K. Barjatiya, S. Dhing, R. Bhatnagar, S. Kothari and V. Dhar, Urol. Res., 2001, 29, 238 CrossRef CAS PubMed; (c) J. J. Clarkson and J. Mc Loughlin, Int. Dent. J., 2000, 50, 119 CrossRef CAS PubMed.
- http://www.who.int/water_sanitation_health/dwq/chemicals/fluoride.pdf.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details; spectra or other electronic format. See DOI: 10.1039/c6ra14287g |
| ‡ Graduate students Jun Qin, Huirong Yao make same contribution for this work. |
| This journal is © The Royal Society of Chemistry 2016 |