Enhanced magnetic properties and MRI performance of bi-magnetic core–shell nanoparticles
Fernando Arteaga Cardona*a,
Esmeralda Santillán Urquizaa,
Patricia de la Presabc,
Silvia Hidalgo Tobónde,
Umapada Palf,
Patricia Horta Fraijog,
Miguel José Yacamang,
José Daniel Lozada Ramíreza,
Robert Ivkovh,
Aracely Angulo-Molinai and
Miguel Ángel Méndez-Rojas*a
aDepartamento de Ciencias Químico-Biológicas, Universidad de las Américas Puebla, 72810 Puebla, Mexico. E-mail: fernando.arteagaca@udlap.mx; miguela.mendez@udlap.mx; Tel: +52 222 4568069 Tel: +52 222 2292607
bInstituto de Magnetismo Aplicado, UCM-ADIF-CSIC, A6 22,500 Km, 28230 Las Rozas, Spain
cDepartamento de Física de Materiales, UCM, Ciudad Universitaria, 28040 Madrid, Spain
dDepartamento de Física, Universidad Autónoma Metropolitana, Avenida San Rafael Atlixco 186 Iztapalapa, Mexico City, Mexico
eDepartamento de Imagenología, Hospital Infantil de México Federico Gómez, Dr Marquez, Col. Doctores, Mexico City, Mexico
fInstituto de Física, Benemérita Universidad Autónoma de Puebla, Apdo. Postal J-48, Puebla, Pue. 72570, Mexico
gDepartment of Physics and Astronomy, University of Texas at San Antonio, One UTSA Circle, San Antonio, TX 78249, USA
hDepartment of Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA
iDepartamento de Ciencias Químico-Biológicas/DIFUS, Universidad de Sonora, Colonia Centro, 83000, Hermosillo, Sonora, Mexico
First published on 10th August 2016
Abstract
Two sets of bi-magnetic Zn0.5Mn0.5Fe2O4@Fe3O4 core–shell nanoparticles were prepared by a seed-mediated modified co-precipitation method. While the first set was obtained by fast addition of the alkaline solution to grow Fe3O4 shells over the ferrite seeds, a slow drop-wise addition of stoichiometric Fe2+/Fe3+ ion solution to the alkaline ferrite seeds solution was adopted to synthesize the second set. Samples were characterized by electron microscopy (STEM, TEM, UHRTEM) and magnetometry measurements. Viability MTT assay of the nanoparticles on L929 murine fibroblasts were performed, indicating that they are biocompatible. The coating of the Zn0.5Mn0.5FeO4 nanoparticle by a magnetite or maghemite shell minimizes the effect of the magnetic dead layer at the core surface, improving the magnetic properties and offering thus outstanding values for biological application. Relaxometry values r2 higher than 300 mM−1 s−1 at H 1.5 T, and cell viability at concentrations as high as 0.5 mg mL−1 means these bi-magnetic nanoparticles have a vast potential as MRI contrast agents.
Introduction
MRI is one of the best non-invasive analytical tools available for clinical diagnosis of several health conditions, as it can produce 2D or 3D images of soft tissues without the use of ionizing radiation, enabling widespread applications.1 MRI contrast agents (CAs) improve sensitivity and diagnosis accuracy. CA materials enhance the detected MR signal by affecting the proton relaxation times of water molecules in their vicinity.2 Biocompatible, nanostructured magnetic CAs, with excellent stability, improved relaxometry properties, and controlled properties are attractive for their potential uses in treatment and diagnosis.3–5 Some nano-CAs have received approval for clinical use, such as Feridex®, Resovist®, Sinerem®, Clariscan® and Lumirem®, with a particle size of 60 to 180 nm range, good biodistribution, and biocompatibility. However, due to different reasons (symptoms such as hypotension, lumber and leg pain, vasodilatation, paresthesia reported with less than 3% of incidence; false positives and safety concerns for some of them) all of them have been withdrawn from markets except Lumirem.6Due to their magnetic properties, iron oxide nanoparticles are potentially useful for therapy, diagnosis, or theranostic applications.7 Recently, the physics of magnetic heat generation for use in magnetic hyperthermia has been recently discussed in detail.8,9 Use of iron oxide nanoparticles as drug delivery agents has also been explored.10 For this purpose, frequently an engineered magnetic nanoparticle (EMN) is loaded with a drug and directed to the target tissue by applying external magnetic field or by fine-tuning the molecular recognition specificity, attaching appropriate molecules on their surfaces.
The MRI contrast (positive, T1-weighted, or negative, T2-weighted) depends on the magnetic properties of the CAs. Materials that produce darker contrast are preferred, as they will require small quantities of the CAs to produce a better image. Although most of the positive T1-weighted CAs are paramagnetic (e.g. gadolinium(III) chelates), superparamagnetic iron oxide nanoparticles are negative T2-weighted CAs and are an attractive option, as they possess large magnetic moments, dipolar interactions between the magnetic cores and surroundings may result in increased longitudinal and transverse relaxation rates, thus enhancing sensitivity.11 Furthermore, reduced size, biocompatibility, stability and solubility in physiological conditions make them appropriate for intravenous injection. Bioconjugation of different molecules such as proteins, antibodies, peptides or sugars on their surface by simple chemistry, makes them interesting for fine-tuning their selectivity.12 While the physical properties of magnetic nanoparticles depend on their preparation conditions, their stability depends on their size and crystallinity. Magnetic response of such particles can be controlled by substitution in the crystal lattice.
In this work, we report that simple changes in the preparation conditions of bi-magnetic core–shell nanoparticles (fast addition of an alkaline solution or slow addition of Fe2+/Fe3+ ions) affect their magnetic properties as well as their performance as MRI CAs. The bi-magnetic system, formed by growing a superparamagnetic disordered Fe3O4 shell on a ferromagnetic ZnMn ferrite crystalline core, will present exchange coupling among the magnetic layers, affecting their magnetic properties. Saturation magnetization and relaxometry values of the core–shell nanoparticles were higher than the values of the core alone, suggesting that engineering the architecture and composition of magnetic core–shell structures may become a simple strategy to enhance and tailor the magnetic properties and the performance of these materials for biomedical applications.13,14
Methods
All the chemicals were purchased from Sigma-Aldrich (Toluca, Mexico). Manganese(II) chloride tetrahydrate (MnCl2·4H2O, >98%), zinc chloride (ZnCl2, >98%), sodium hydroxide (NaOH, >97%), iron(II) chloride tetrahydrate (FeCl2·4H2O, >98%), iron(III) chloride hexahydrate (FeCl3·6H2O, >97%), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, >98%), hydrochloric acid (HCl, 37%) and nitric acid (HNO3, 70%) were reagent grade and used as delivered without further purification, unless stated otherwise. Magnetic nanoparticles (MNPs) were obtained by co-precipitation, a well-known preparation method.15 Stabilization of the fabricated nanoparticles was achieved by acid peptization in aqueous media to reduce the potential aggregation/disaggregation.16,17Highly stable, water soluble, non-aggregating core–shell MNPs (10–16 nm) were obtained in aqueous solution by a two-step process. Aqueous synthesis procedures are preferable for the preparation of materials to be used in biomedical applications, as they minimize contamination with toxic reagents and bulk production can be easily achieved by scaling the preparation method. Briefly, Zn0.5Mn0.5Fe2O4 ferrite (ZnMn) nanoparticles were prepared following previously reported procedures.18–21 In a second step, ZnMn nanoparticles were used as nucleation seeds to obtain core–shell bi-magnetic nanoparticles. Two variants of this step allowed the preparation of two different sets of products. The first one was achieved by the fast addition of alkaline NaOH solution to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Fe2+/Fe3+ mixture, in the presence of the seeding ZnMn nanoparticles. The second variation consisted of the slow drop-wise addition of a 1
2 Fe2+/Fe3+ mixture, in the presence of the seeding ZnMn nanoparticles. The second variation consisted of the slow drop-wise addition of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Fe2+/Fe3+ mixture into the alkaline dispersion of ZnMn ferrite seeds. After the completion of addition solution process, the shell forming reaction was continued for 30 minutes or two hours before it was quenched for both systems. Table 1 summarizes the preparation conditions used for each set of products. It must be emphasized that the intent of this work is to obtain and characterize core–shell nanoparticles by aqueous solution-based methods in order to ascertain their potential utility for biomedical applications.
2 Fe2+/Fe3+ mixture into the alkaline dispersion of ZnMn ferrite seeds. After the completion of addition solution process, the shell forming reaction was continued for 30 minutes or two hours before it was quenched for both systems. Table 1 summarizes the preparation conditions used for each set of products. It must be emphasized that the intent of this work is to obtain and characterize core–shell nanoparticles by aqueous solution-based methods in order to ascertain their potential utility for biomedical applications.
| Sample name | Chemical composition | Shell reaction time | Mode of addition of Fe ions for shell formation |
|---|---|---|---|
| Fe3O4 | Fe3O4 | — | — |
| ZnMn | Zn0.5Mn0.5Fe2O4 | — | — |
| F30m | Zn0.5Mn0.5Fe2O4–Fe3O4 | 30 minutes | Fast |
| F2h | Zn0.5Mn0.5Fe2O4–Fe3O4 | 2 hours | Fast |
| D30m | Zn0.5Mn0.5Fe2O4–Fe3O4 | 30 minutes | Drop-wise |
| D2h | Zn0.5Mn0.5Fe2O4–Fe3O4 | 2 hours | Drop-wise |
Synthesis of Fe3O4 (Fe3O4)
Magnetite nanoparticles were prepared as follows: 2.5 mmol of FeCl2·4H2O was dissolved in 1 mL of distilled water with 250 μL of HCl (37%). Iron(III) chloride hexahydrate (5 mmol) was dissolved in 5 mL of distilled water, and added to the Fe2+ solution; then the solution containing both Fe2+ and Fe3+ was rapidly added to a NaOH 1 M solution (50 mL). The mixture was stirred for 30 minutes to form magnetite (Fe3O4), a black precipitate that was magnetically decanted and washed three times with distilled water.Synthesis of Zn0.5Mn0.5Fe2O4 (ZnMn)
To synthesize the manganese–zinc ferrite 1.2 mmol of ZnCl2 and 1.2 mmol of MnCl2·4H2O were dissolved in 1 mL of distilled water and 250 μL of HCl (37%). Iron(III) chloride hexahydrate (5 mmol) was dissolved in 5 mL of distilled water, and added to the mixture of manganese and zinc ions, then the solution containing the salts was rapidly poured into a 1 M NaOH solution (50 mL) at 100 °C. The mixture was stirred for 30 minutes, and the product was separated by magnetic decantation, washing several times with distilled water.Synthesis of Zn0.5Mn0.5Fe2O4@Fe3O4 by fast addition of Fe2+/Fe3+ salts (Ft, t = 30 m or 2 h)
Previously prepared ferrite ZnMn (0.5 g, 2.12 mmol) was dispersed in 10 mL of water and then a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dissolved Fe2+/Fe3+ ions was added (for t = 30 m, this mixture was prepared as described for the synthesis of Fe3O4 nanoparticles; for t = 2 h, the concentration of Fe2+/Fe3+ ions was doubled). After the addition had been completed, 40 mL of an aqueous 1.25 M NaOH solution was rapidly added to the reaction mixture. Finally, the black precipitate was magnetically decanted and washed several times with distilled water.
1 mixture of dissolved Fe2+/Fe3+ ions was added (for t = 30 m, this mixture was prepared as described for the synthesis of Fe3O4 nanoparticles; for t = 2 h, the concentration of Fe2+/Fe3+ ions was doubled). After the addition had been completed, 40 mL of an aqueous 1.25 M NaOH solution was rapidly added to the reaction mixture. Finally, the black precipitate was magnetically decanted and washed several times with distilled water.
Synthesis of Zn0.5Mn0.5Fe2O4@Fe3O4 by slow addition of Fe2+/Fe3+ salts (Dt, t = 30 m or 2 h)
Previously prepared ferrite ZnMn (0.5 g, 2.12 mmol) was dispersed in 50 mL of a 1 M NaOH solution. Then, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Fe2+/Fe3+ ions was slowly added drop-wise at a rate of 100 μL min−1. The concentration of the mixture of iron ions was as previously described for t = 30 m or t = 2 h. Finally, the black powder was separated by magnetic decantation and washed several times with distilled water.
1 mixture of Fe2+/Fe3+ ions was slowly added drop-wise at a rate of 100 μL min−1. The concentration of the mixture of iron ions was as previously described for t = 30 m or t = 2 h. Finally, the black powder was separated by magnetic decantation and washed several times with distilled water.
Aqueous stabilization by acid peptization
Briefly, 25 mL of 2 M HNO3 was added to the washed MNPs and stirred for 15 minutes. Then, the aqueous acidic supernatant was magnetically decanted, and 5 mL of iron(III) nitrate nonahydrate (1 M) solution and 20 mL of water were added and boiled for 25 minutes. After that time, the supernatant was magnetically decanted. Finally, 25 mL of 2 M HNO3 was added to the sample and left stirring for 15 minutes, then the product was separated by magnetic decantation and washed with acetone. Finally, samples were re-dispersed in water.Characterization
Particle and aggregate size
Particle size and shape of the synthesized nanostructures were analyzed using a JEOL JEM1010, a JEOL 1230 and an aberration-corrected JEOL ARM 200F ultra-high resolution transmission electron microscope, UHRTEM (JEOL USA, Inc., Peabody, MA) located at the ICMM-CSIC and the Kleberg Advanced Microscopy Center-UTSA, respectively. Scanning transmission electron microscope (STEM) images were acquired using a Hitachi STEM S5500 (Hitachi High-Technologies Co., Tokyo, JP) equipped with an energy-dispersive spectroscopy (EDS) detector. The average particle size was measured by counting more than 200 particles of each sample individually and fitted with a lognormal distribution to calculate the mean size and the standard deviation. Size dispersion and the hydrodynamic diameter of the samples were measured at room temperature using dynamic light scattering (DLS) instrument NanoFlex (Microtrac Inc., Montgomeryville, PA, USA), with a 780 nm wavelength red laser of 3 mW.XRD analysis
X-ray diffraction (XRD) patterns were measured by Powder Diffractometre Bruker D8 Advance with Cu Kα radiation with energy-discriminator (solx). The X-ray patterns were collected between 15° and 80° of 2θ.Chemical composition
Determination of ratio of Mn(II), Zn(II) and total Fe ions was carried out using an inducing coupling plasma optical emission spectrometer (ICP-OES), OPTIME 2100DV from Perkin Elmer. The wavelengths used to determine the transition metal ions were 257.61, 206.2 and 238.20 nm. Since some elements can interfere with the iron measurements, another wavelength was used (239.56 nm) to have a higher precision for the evaluation of iron contain. For this purpose, 50 μL of the samples were digested by using a solution of HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCl (3
HCl (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The concentration was adjusted to be 10 ppm, and the values of intensity were compared to a previously made calibration curve to obtain the mass of each ion. The results were adjusted to the Mn mass, considering the stoichiometry for the ZnMn ferrite (Zn0.5Mn0.5Fe2O4). Table 2 shows the adjusted number of moles according to the ICP-OES analysis for samples F30m and F2h; it can be noted that the iron concentration increases when the Fe3O4 shell formation reaction passes from 30 min to 2 h. This increase can be attributed to a thicker magnetite shell growing over the ZnMn nanoparticle core.
1). The concentration was adjusted to be 10 ppm, and the values of intensity were compared to a previously made calibration curve to obtain the mass of each ion. The results were adjusted to the Mn mass, considering the stoichiometry for the ZnMn ferrite (Zn0.5Mn0.5Fe2O4). Table 2 shows the adjusted number of moles according to the ICP-OES analysis for samples F30m and F2h; it can be noted that the iron concentration increases when the Fe3O4 shell formation reaction passes from 30 min to 2 h. This increase can be attributed to a thicker magnetite shell growing over the ZnMn nanoparticle core.
| Sample | Mn | Zn | Fe (core) | Fe (shell) |
|---|---|---|---|---|
| F30m | 0.50 | 0.37 | 2.00 | 3.90 |
| F2h | 0.50 | 0.52 | 2.00 | 8.98 |
Magnetic characterization
Hysteresis curves (room temperature and 10 K) of the samples were recorded in a magnetic field up to 5 T for hysteresis curves. Zero field cooled–field cooled (ZFC–FC) measurements were done with a magnetic field of 200 Oe. A vibrating sample magnetometer (VSM) attached to a Physical Property Measurements System (PPMS, Quantum Design, Dyna-cool 9, USA) was used for all the magnetic measurements by placing the sample into tubular plastic sample holders. The approximate magnetic diameters of the samples at 300 K were calculated using Chanterell's equation (eqn (1)):22
 | (1) |
Performance as MRI contrast agents
Relaxometry of the samples was determined in a clinical 1.5 T MRI scanner (Philips Intera-Achieva (Philips Healthcare, Best, Netherlands)). Samples were dispersed in a 6.6 mg mL−1 agar solution; the concentration of the samples was adjusted to 0.025, 0.050, 0.075, 1.000 and 1.025 mg in 15 mL plastic tubes. The r2 values were calculated using a multi-fast field echo sequence (mFFE) from 4.6 to 104.6 ms TE; intensity contrast of the images was obtained using OsiriX software (Pixmeo, Geneva, Switzerland).23 For r2 calculation, first the data were adjusted to an exponential decay equation (eqn (2)), and then the rate values (R2 = 1/T2) were plotted against the total ion concentration of the samples in mM to obtain the relaxometry (r2) values from the slope of the graph:
 | (2) |
Cell culture
Cell line L929 (mouse fibroblast derived from adipose tissue) obtained from the American Type Culture Collection (ATCC, Rockville, MD) was used in this study. The cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 1% penicillin–streptomycin, and 1% glutamine at 37 °C and 5% CO2 atmosphere during 24 h. Cells were seeded in triplicate at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 on 96-well culture plates and incubated for 24 h before the experiments.24
000 on 96-well culture plates and incubated for 24 h before the experiments.24
In vitro viability assay
The L929 cells were exposed to different concentrations of nanoparticles previously suspended in culture media and serially diluted (0–1000 μg mL−1). For measuring cell viability after exposition to the prepared nanoparticles, a MTT (3-[4,5-dimethylthyazol-2-yl]-2,5 diphenyl tetrazolium bromide) colorimetric assay was utilized at 24 and 48 h. After the incubation period, sample solutions were removed, rinsed with PBS and MTT solution (5 mg mL−1 in PBS pH 7.4) was added to all the wells and incubated for 4 h at 37 °C in the dark. Then, 100 μL of 0.004 N HCl in isopropanol was added to each well to the lysate. Absorbance was monitored in a microplate reader at a wavelength of 550 nm. Untreated cells were considered as controls.24 The cell viability was calculated as:| % cell viability = (absorbance of sample well/absorbance of control well) × 100 |
Analysis of results was done with the program GraphPad Prism 5 for the calculation of viability curves. Results were expressed as mean values ± SEM (standard error of the mean) in triplicate.
Results and discussion
The methods used to prepare core–shell MNPs in this work are based on colloidal wet chemistry, which has been widely studied.25,26 Eqn (3)–(5), describe the chemical reactions that yield the magnetic materials fabricated in this study. Water soluble, highly stable, non-aggregating MNPs could be obtained with an excellent control of size and size dispersity, as shown in Table 2.| Fe2+(aq) + 2Fe3+(aq) + 8OH−(aq) → Fe3O4(s) + 4H2O(l) | (3) |
| 0.5Zn2+(aq) + 0.5Mn2+(aq) + 2Fe3+(aq) + 8OH−(aq) → Zn0.5Mn0.5Fe2O4(s) + 4H2O(l) | (4) |
| Zn0.5Mn0.5Fe2O4(s) + Fe2+(aq) + 2Fe3+(aq)+ 8OH−(aq) → Zn0.5Mn0.5Fe2O4@Fe3O4(s) + 4H2O(l) | (5) |
Structural characterization
Fig. 1 presents the TEM images and size distribution histograms of the samples. As expected, core–shell MNPs have larger sizes than the first ZnMn nanoparticles used as seeds, as well as different polydispersity. Table 3 summarizes the results of average nanoparticle sizes as determined by several methods (TEM, DLS, and magnetic measurements), as well as polydispersity index (PI). The sample F30m shows the highest polydispersity in comparison to the other samples. This increase in the polydispersity may indicate that the process of shell formation competes with the nucleation process, producing a more random and polydisperse shell structure.20 After 2 hours of shell formation, the polydispersity decreases to 0.19 and the nanoparticle size increases to 12.3 nm. In contrast, slow, drop-wise addition of the Fe2+/Fe3+ mixture leads to a lower polydispersity and a larger particle size (see Table 3). The fast addition of the Fe2+/Fe3+ mixture may create a disordered and non-uniform shell, composed of smaller particles compared to a more controlled and uniform layers due to the slow drop-by-drop addition of precursors. This observation is consistent with previously published findings, where the effect of the fast or slow addition of the alkaline solution on the formation of magnetite was studied.27 In that work, smaller magnetite nanoparticles were obtained by fast addition of a NaOH solution, in contrast to those obtained by slow addition of the base. The degree of polydispersity and crystallinity of growing crystallites were affected by physical variables such as temperature, concentration of reagents, quenching reaction time and reagent addition rate.28 The rate of addition of the base solution affects the ordering (crystallinity) of the magnetic shell layer forming on the surface of the ZnMn nanoparticles used as seeds, which in turns affects the magnetic properties.20 All samples present low polydispersity (Table 3), the highest polydispersity index (PI) value being 0.24 for sample F30m, well below 0.3, the limiting PI value recommended for biomedical applications.29 | ||
| Fig. 1 TEM and STEM images of samples (a) Fe3O4, (b) ZnMn, (c) F30m, (d) F2h, (e) D30m and (f) D2h with their size distribution fitted using a lognormal function. | ||
| Sample | DTEM (nm) | DH (nm) | PI (DH) | Dmag (nm) |
|---|---|---|---|---|
| a PI = SD/mean. | ||||
| Fe3O4 | 6.4 ± 0.2 | 23.2 ± 7.9 | 0.34 | 5.6 |
| ZnMn | 8.0 ± 0.2 | 20.3 ± 7.7 | 0.38 | 6.3 |
| F30m | 10.0 ± 0.3 | 27.0 ± 11.1 | 0.41 | 8.8 |
| F2h | 12.3 ± 0.2 | 28.6 ± 9.1 | 0.32 | 7.8 |
| D30m | 11.5 ± 0.2 | 29.0 ± 9.8 | 0.34 | 10.5 |
| D2h | 15.6 ± 0.1 | 25.0 ± 6.5 | 0.26 | 8.7 |
Fig. 2a, UHRTEM image of sample D2h, clearly shows a close packed cubic structure. Fe3O4 is a ferromagnetic material which crystallizes within a spinel cubic structure, so these images are in agreement with the expected for a magnetite shell. The corresponding FFT pattern in Fig. 2b arise from the adjacent region in the shell; the image clearly shows the structure of the shell, but the core can't be solved due to probable different crystal orientation.47 XRD analyses (Fig. 5) confirmed that the nanoparticles are in the nanocrystalline cubic spinel phase. The noisy XRD diffractograms for samples F30m and D30m suggest that the Fe3O4 shell formed on the surface of the ZnMn ferrite core is disordered or amorphous. These results are similar to those reported by Song and coworkers for a series of magnetically active ferrite NPs prepared by growing a Fe3O4 shell on Mn oxide NPs used as seeds.27
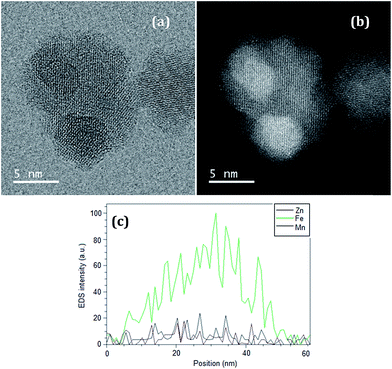
Fig. 3 presents TEM images for a selected nanoparticle from sample D2h, in bright (Fig. 3a) and dark (Fig. 3b) fields. EDS analysis along a horizontal line crossing through the particle (Fig. 3c) suggests a composition that resembles the expected core–shell structure, based on the changes in Fe, Zn, and Mn concentration along the length of measurement. We note that UHRTEM images do not enable us to distinguish clearly the core from the shell in the Fe3O4/ZnMn system, as the magnetite shell grows over the ZnMn ferrite core, and both have similar crystal structures. On the other hand, EDS data (Fig. 3c) and information obtained from ICP (Table 2) and XRD (Fig. 5) suggest that a disordered core–shell structure was achieved by this modified co-precipitation method. The synthetic methodology to get these nanostructured systems through the modified co-precipitation of a magnetite shell on the ZnMn seed/core under different conditions especially the rate of precursor addition seems to play a major role in achieving these highly disordered core–shell structures. The shell may be composed by nanocrystallites growing on the core surface, as Fig. 3a and b seem to indicate.
As can be seen from Fig. 4, the hydrodynamic size particles are smaller than 35 nm, suggesting well-dispersed nanoparticles as expected for non-aggregated samples stabilized by acid peptization. It has been reported that the MRI performance may be affected by the use of polymeric coatings as surfactants,30 due to a variety of inter-particle weak interactions that may induce aggregation and dipolar interactions between particles.31
 | ||
| Fig. 4 Particle size distribution of aqueous suspensions of magnetic and bi-magnetic core–shell nanoparticles determined by DLS. | ||
XRD diffraction patterns do not show any significant difference between samples ZnMn and Fe3O4 with respect to the core–shell samples, as both the core and the shell have the same crystal structure. Although both crystal structures are the same (mixed spinel) and have different interplanar distances because of their different composition, the resulting diffraction peaks become broader compared to the pure Fe3O4 or ZnMn samples (Fig. 5). This effect is probably due to the loss of long range ordering at the particle surface.
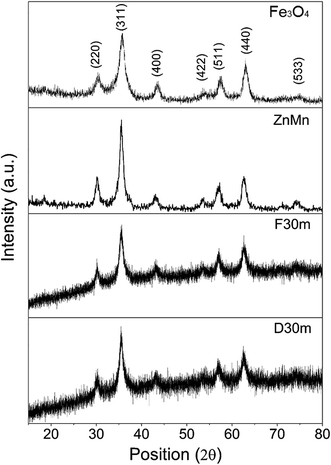 | ||
| Fig. 5 XRD patterns of the core and shell alone (top), and the bi-magnetic core–shell structures D30m and F30m (bottom). | ||
Magnetic characterization
As expected for a core–shell structure, the calculated magnetic size of the samples, shown in Table 4, are smaller than the particle size determined by TEM. This decrease in size is a consequence of interfacial defects such as a magnetic dead layer (MDL) (originated by vacancies, dangling bonds, and disorder on the layer) which diminishes the effective volume of the MNPs contributing to the magnetism.27,32 Moreover, that calculated magnetic size is just a theoretical approximation to the real size of the magnetic contribution of the nanoparticles.33,34| Samples | Msat (emu g−1) | Msat% increase | TB (K) | Hc (Oe) at 10 K | ||
|---|---|---|---|---|---|---|
| 300 K | 10 K | 300 K | 10 K | |||
| Fe3O4 | 37.72 | 47.21 | — | — | 112 | 271 |
| ZnMn | 42.37 | 77.22 | — | — | 123 | 150 |
| F30m | 49.21 | 71.38 | 16.14% | −7.56% | 219 | 164 |
| F2h | 53.13 | 71.04 | 25.39% | −8.00% | 168 | 184 |
| D30m | 43.65 | 68.05 | 3.02% | −11.87% | 176 | 260 |
| D2h | 50.69 | 66.29 | 19.63% | −14.15% | 217 | 250 |
One of the most surprising results of this work is the increase by 25% of saturation magnetization of the core–shell structure in F2h regarding the corresponding individual values of the ZnMn (core) or Fe3O4 (shell) (Table 4). This improvement is observed especially at room temperature, which is the temperature range of interest for any biological application.
On the other hand, the systems obtained by slow, dropwise addition of the Fe2+/Fe3+ mixture have small increments of Ms, just about 3% and 19% after 30 minutes and 2 hours of synthesis, respectively. In comparison, core–shell MNPs obtained by the fast addition of alkaline solution method presented increments of 18% and 25%, after 30 minutes and 2 hours, respectively.
For the core–shell systems, the Ms at 10 K are lower than the corresponding value for the ZnMn ferrite core. The saturation magnetization decreases up to 14% in the core–shell systems with respect to ZnMn; however, their Ms at 300 K are nearly 25% higher. The existence of a shell with antiparallel spins (with respect to the core spins) dispersed in the system might be responsible for this behavior at low temperature. Although thermal fluctuations may be sufficient to allow these antiparallel spins to shift and reverse partially to each other at 300 K, at 10 K the heat energy is insufficient to induce these thermal fluctuations, and the shell spins align antiparallel to the core spins diminishing the values of Ms in the core–shell systems. The later may explain the differences observed in the hysteresis curves at 300 K and 5 K (insets, Fig. 6). It has been previously reported that competing interactions among magnetic layers or uncompensated spins at the interface in core–shell systems may induce the spins to be antiferromagnetically aligned, resulting on net magnetization and affecting the Ms values.35,36
Exchange coupling interactions between magnetic layers may explain the enhancement in Ms at 300 K. Increments and variations of the magnetic properties of bi-magnetic structures has been reported previously for ferromagnetic/antiferromagnetic (FM/AFM) and antiferromagnetic/ferromagnetic (AFM/FM) core–shell systems.37–40
The differences in magnetic behavior among the core–shell samples obtained by both methods may be explained by the exchange coupling between the magnetic core and the magnetic shell. The spin coupling arises from the effect of dipolar interactions between the magnetic core and the shell.41 It is well known that the dipolar effect has a significant influence on the magnetic properties of materials. For bi-magnetic core–shell systems, the whole core is in full contact with the shell, while the surrounding MNPs may also contribute to these magnetic interactions. Eqn (6) clearly state that the energy of the dipole changes by increasing the magnetic moment and decreasing the interparticle distance.42
 | (6) |
The changes observed in magnetic properties for the two different sets of magnetic core–shell nanoparticles suggest that the slow addition of the iron salts may produce a thick MDL, which decreases the exchange coupling between the core and the shell. On the other hand, the fast addition of alkaline solution seems to favor the rapid nucleation of magnetite on the seed surface, creating a thin MDL, decreasing the distance between the magnetic layers, and increasing the MNP polydispersity. After 30 min or 2 h of reaction, homogenization of the shell thickness occurs in both cases, with no significant change in their magnetic properties (Table 4). As the shell becomes thinner, the effective core–shell interface becomes larger and the exchange coupling increases.43
Blocking temperature (TB) was approximated from the ZFC–FC curves (Fig. 7), and values are shown in Table 4. The TB values remain significantly low suggesting that the MNPs may flip their spins, reversing their net magnetization at room temperature and above. The reversal of magnetization might be of fundamental importance as heat generation in magnetic hyperthermia occurs by Néel relaxation.
Performance as MRI T2 contrast
A 1.5 T MRI instrument was used to measure the signal decay. The samples total mass concentrations were adjusted to be from 0.025 mg to 1.025 mg. Fig. 8 shows the exponential decay of the signal of water, the higher the concentration, the faster the signal decay is. Zero concentration in some cases adopts what it may seem a linear decay. Rate (R2 = 1/T2) values were acquired by fitting decay of the intensity signal to a monoexponential decay (STE = S0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−TE/T2),44 and rate values were plotted against the concentration values giving a linear response.45 The slopes of the fitted linear plots (Fig. 9) are the relaxation values (r2). Relaxation values of the samples are summarized in Table 5.
e−TE/T2),44 and rate values were plotted against the concentration values giving a linear response.45 The slopes of the fitted linear plots (Fig. 9) are the relaxation values (r2). Relaxation values of the samples are summarized in Table 5.
 | ||
| Fig. 9 Linear relationship of R2 values plotted against the concentration of total ions used to calculate the relaxometry (r2) values for all the samples. | ||
| Sample | r2 (mM−1 s−1) | % increase |
|---|---|---|
| ZnMn | 262.62 | — |
| F30m | 323.05 | 23.01 |
| F2h | 386.61 | 47.21 |
| D30m | 194.91 | −25.78 |
| D2h | 242.55 | −7.64 |
Although an agglomeration of nanoparticles is known to increase the r2 signal intensity,46 our systems can be considered monodispersed (as seen from the DLS analysis) and have no significant agglomeration (Fig. 4).
Sample F2h has the highest r2 value among all samples. It is interesting to note that the sample with the highest Ms value also has the highest r2 indicating that the field inhomogeneities introduced by the MNPs have a higher dependency to the Ms values of the magnetic materials. The increase in the r2 value of sample F2h was almost 50% respect to the core ZnMn sample. On the other hand, the samples synthesized by slow addition decreased the r2 values 25% in both cases (at 30 minutes and 2 hours), even though the slow addition D30 samples had higher Ms values than ZnMn. This reduction in the r2 values was completely unexpected and other parameters, aside of Ms and aggregation, also played a crucial part in making the precession movement of the water protons slower. The later suggest that the T2 contrast performance of all core–shell samples may be optimized by controlling aggregation and fine-tuning of their Ms values primarily.
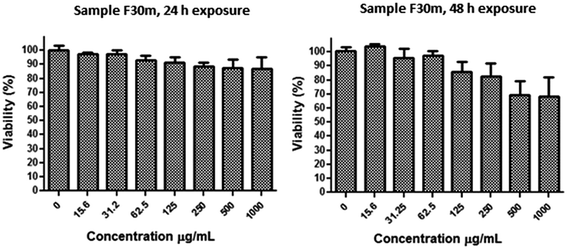
Cell culture and in vitro viability assay
Conclusion
In summary, we demonstrate that different preparation pathways (fast addition of NaOH or slow, drop-wise addition of iron precursors) can induce significant differences in the magnetic properties of core–shell bi-magnetic nanoparticles, affecting considerably their Ms values and relaxitivity, which determine their MRI performance as contrast agents. Fast addition of alkaline solution produces bi-magnetic core–shell nanoparticles of superior magnetic properties with respect to those of magnetite cores. While the core–shell nanostructures prepared by fast addition of alkaline solution and 30 min reaction time (sample D30m) have 25% higher Ms values than the core alone, the sample prepared at 2 h reaction time (sample D2h) has better r2 value, indicating an improved MRI performance than the other systems. Also, all the nanoparticles presented good responses under using a 1.5 T MRI scan, which is the most common MRI equipment that hospitals have. For the samples synthesized by slow addition of iron ions, the variations in magnetic properties were modest. These differences can be attributed to a magnetic exchange interaction between the core and the shell, suggesting that changes in the structure of the interface between the core and the shell may play a significant role in the exchange interactions. The MDL could get thicker or thinner, depending on the synthetic conditions, affecting the exchange interaction between the magnetic layers. To maximize this interaction between the magnetic layers, the preparation methodology for bi-magnetic core–shell nanostructures must produce a disordered interface as thin as possible, favoring a more efficient exchange coupling between the spins of the core and the shell. Performed in vitro cell viability assays indicate that the core–shell nanoparticles are biocompatible, with no adverse effect on cells for concentrations up to 500 μg mL−1. The usefulness of the fabricated bi-magnetic nanostructures as MRI contrast agent under available clinical conditions (using a 1.5 T scanner) has been demonstrated. Further work on the development of bi-magnetic core–shell systems useful as MRI contrast agent, and for hyperthermal therapy is in progress.Acknowledgements
The work was supported by CONACYT (Grants # CB-2010/154602, CB-2010/151767, and INFR-2014-02-230530) and the Spanish Ministry of Science and Innovation (MAT2012-37109-C02-01). SHT thanks, Hospital Infantil de México Federico Gómez for access to its clinical MRI facilities.References
- H. Shokrollahi, A. Khorramdin and G. Isapour, Magnetic Resonance Imaging by Using Nano-Magnetic Particles, J. Magn. Magn. Mater., 2014, 369, 176–183 CrossRef CAS
.
- M. Wabler, W. Zhu, M. Hedayati, A. Attaluri, H. Zhou, J. Mihalic, A. Geyh, T. L. DeWeese, R. Ivkov and D. Artemov, Magnetic Resonance Imaging Contrast of Iron Oxide Nanoparticles Developed for Hyperthermia Is Dominated by Iron Content, Int. J. Hyperthermia, 2014, 30, 192–200 CrossRef CAS PubMed
.
- D. K. Kim, Y. Z. J. Kehr, T. Klason, B. Bjelke and M. Muhammed, Characterization and MRI Study of Surfactant-Coated Superparamagnetic Nanoparticles Administered into the Rat Brain, J. Magn. Magn. Mater., 2001, 225, 256–261 CrossRef CAS
.
- H. Hejase, S. S. Hayek, S. Qadri and Y. Haik, MnZnFe Nanoparticles for Self-Controlled Magnetic Hyperthermia, J. Magn. Magn. Mater., 2012, 324, 3620–3628 CrossRef CAS
.
- M. Levy, C. Wilhelm, J. M. Siaugue, O. Horner, J. C. Bacri and F. Gazeau, Magnetically Induced Hyperthermia: Size-Dependent Heating Power of Gamma-Fe2O3 Nanoparticles, J. Phys.: Condens. Matter, 2008, 20, 204133 CrossRef PubMed
.
- Y.-X. J. Wang, Superparamagnetic Iron Oxide Based MRI Contrast Agents: Current Status of Clinical Application, Quant. Imag. Med. Surg., 2011, 1, 35–40 Search PubMed
.
- D. Yoo, J.-H. Lee, T.-H. Shin and J. Cheon, Theranostic Magnetic Nanoparticles, Acc. Chem. Res., 2011, 44, 863–874 CrossRef CAS PubMed
.
- D. Ortega and Q. A. Pankhurst, Magnetic Hyperthermia, in Nanoscience: Volume 1: Nanostructures through Chemistry, The Royal Society of Chemistry, 2013, vol. 1, pp. 60–88 Search PubMed
.
- C. L. Dennis and R. Ivkov, Physics of Heat Generation Using Magnetic Nanoparticles for Hyperthermia, Int. J. Hyperthermia, 2013, 29, 715–729 CrossRef PubMed
.
- X. Mou, Z. Ali, S. Li and N. He, Applications of Magnetic Nanoparticles in Targeted Drug Delivery System, J. Nanosci. Nanotechnol., 2015, 15, 54–62 CrossRef CAS PubMed
.
- M. P. Morales, O. Bomati-Miguel, R. Pérez de Alejo, J. Ruiz-Cabello, S. Veintemillas-Verdaguer and K. O'Grady, Contrast Agents for MRI Based on Iron Oxide Nanoparticles Prepared by Laser Pyrolysis, J. Magn. Magn. Mater., 2003, 266, 102–109 CrossRef CAS
.
- E. Santillán-Urquiza, F. Arteaga-Cardona, E. Hernandez-Herman, P. F. Pacheco-García, R. González-Rodríguez, J. L. Coffer, M. E. Mendoza-Alvarez, J. F. Vélez-Ruiz and M. A. Méndez-Rojas, Inulin as a Novel Biocompatible Coating: Evaluation of Surface Affinities toward CaHPO4, α-Fe2O3, ZnO, CaHPO4@ZnO and α-Fe2O3@ZnO Nanoparticles, J. Colloid Interface Sci., 2015, 460, 339–348 CrossRef PubMed
.
- P. Crespo, P. de la Presa, P. Marin, M. Multigner, J. M. Alonso, G. Rivero, F. Yndurain, J. M. Gonzalez-Calbet and A. Hernando, Magnetism in Nanoparticles: Tuning Properties with Coatings, J. Phys.: Condens. Matter, 2013, 25, 484006 CrossRef PubMed
.
- V. Dupuis, V. Gavrilov-Isaac, S. Neveu, M. Aouadi and S. Abramson, Synthesis and Properties of Magnetic Nanoparticles with Tunable Magnetic Anisotropy Energy, Mater. Res. Soc. Symp. Proc., 2014, 1708, 1708 CrossRef
.
- M. Mascolo, Y. Pei and T. Ring, Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large Ph Window with Different Bases, Materials, 2013, 6, 5549–5567 CrossRef CAS
.
- E. Auzans, D. Zins, E. Blums and R. Massart, Synthesis and Properties of Mn–Zn Ferrite Ferrofluids, J. Mater. Sci., 1999, 34, 1253–1260 CrossRef CAS
.
- F. Arteaga-Cardona, K. Rojas-Rojas, R. Costo, M. A. Mendez-Rojas, A. Hernando and P. de la Presa, Improving the Magnetic Heating by Disaggregating Nanoparticles, J. Alloys Compd., 2016, 663, 636–644 CrossRef CAS
.
- T. Ahn, J. H. Kim, H.-M. Yang, J. W. Lee and J.-D. Kim, Formation Pathways of Magnetite Nanoparticles by Coprecipitation Method, J. Phys. Chem. C, 2012, 116, 6069–6076 CAS
.
- C. Ravikumar and R. Bandyopadhyaya, Mechanistic Study on Magnetite Nanoparticle Formation by Thermal Decomposition and Coprecipitation Routes, J. Phys. Chem. C, 2011, 115, 1380–1387 CAS
.
- N. T. K. Thanh, N. Maclean and S. Mahiddine, Mechanisms of Nucleation and Growth of Nanoparticles in Solution, Chem. Rev., 2014, 114, 7610–7630 CrossRef CAS PubMed
.
- R. Massart, Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media, IEEE Trans. Magn., 1981, 17, 1247–1248 CrossRef
.
- R. Chantrell, J. Popplewell and S. Charles, Measurements of Particle Size Distribution Parameters in Ferrofluids, IEEE Trans. Magn., 1978, 14, 975–977 CrossRef
.
- A. Rosset, L. Spadola and O. Ratib, Osirix: An Open-Source Software for Navigating in Multidimensional Dicom Images, J. Digit. Imag., 2004, 17, 205–216 CrossRef PubMed
.
- A. Angulo-Molina, M. Á. Méndez-Rojas, T. Palacios-Hernández, O. E. Contreras-López, G. A. Hirata-Flores, J. C. Flores-Alonso, S. Merino-Contreras, O. Valenzuela, J. Hernández and J. Reyes-Leyva, Magnetite Nanoparticles Functionalized with α-Tocopheryl Succinate (α-TOS) Promote Selective Cervical Cancer Cell Death, J. Nanopart. Res., 2014, 16, 1–12 CrossRef CAS
.
- C. Pereira, A. M. Pereira, C. Fernandes, M. Rocha, R. Mendes, M. P. Fernandez-Garcia, A. Guedes, P. B. Tavares, J.-M. Grenèche, J. P. Araujo and C. Freire, Superparamagnetic MFe2O4 (M = Fe, Co, Mn) Nanoparticles: Tuning the Particle Size and Magnetic Properties through a Novel One-Step Coprecipitation Route, Chem. Mater., 2012, 24, 1496–1504 CrossRef CAS
.
- P. Tartaj, M. d. P. Morales, S. Veintemillas-Verdaguer, T. González-Carreño and C. J. Serna, The Preparation of Magnetic Nanoparticles for Applications in Biomedicine, J. Phys. D: Appl. Phys., 2003, 36, R182–R197 CrossRef CAS
.
- H.-M. Song, J. I. Zink and N. M. Khashab, Seeded Growth of Ferrite Nanoparticles from Mn Oxides: Observation of Anomalies in Magnetic Transitions, Phys. Chem. Chem. Phys., 2015, 17, 18825–18833 RSC
.
- G. Muralidharan, L. Subramanian, S. K. Nallamuthu, V. Santhanam and S. Kumar, Effect of Reagent Addition Rate and Temperature on Synthesis of Gold Nanoparticles in Microemulsion Route, Ind. Eng. Chem. Res., 2011, 50, 8786–8791 CrossRef CAS
.
- R. P. Araújo-Neto, E. L. Silva-Freitas, J. F. Carvalho, T. R. F. Pontes, K. L. Silva, I. H. M. Damasceno, E. S. T. Egito, A. L. Dantas, M. A. Morales and A. S. Carriço, Monodisperse Sodium Oleate Coated Magnetite High Susceptibility Nanoparticles for Hyperthermia Applications, J. Magn. Magn. Mater., 2014, 364, 72–79 CrossRef
.
- B. Issa, S. Qadri, I. M. Obaidat, R. W. Bowtell and Y. Haik, Peg Coating Reduces Nmr Relaxivity of Mn0.5Zn0.5Gd0.02Fe1.98O4 Hyperthermia Nanoparticles, J. Magn. Reson. Imag., 2011, 34, 1192–1198 CrossRef PubMed
.
- B. Bittova, J. P. Vejpravova, M. P. Del Morales, A. G. Roca, D. Niznansky and A. Mantlikova, Influence of Aggregate Coating on Relaxations in the Systems of Iron Oxide Nanoparticles, Nano, 2012, 07, 1250004 CrossRef
.
- R. H. Kodama, Magnetic Nanoparticles, J. Magn. Magn. Mater., 1999, 200, 359–372 CrossRef CAS
.
- V. Russier, C. de Montferrand, Y. Lalatonne and L. Motte, Size and Polydispersity Effect on the Magnetization of Densely Packed Magnetic Nanoparticles, J. Appl. Phys., 2012, 112, 073926 CrossRef
.
- W. Wei, W. Zhaohui, Y. Taekyung, J. Changzhong and K. Woo-Sik, Recent Progress on Magnetic Iron Oxide Nanoparticles: Synthesis, Surface Functional Strategies and Biomedical Applications, Sci. Technol. Adv. Mater., 2015, 16, 023501 CrossRef
.
- T. E. Torres, E. Lima, A. Mayoral, A. Ibarra, C. Marquina, M. R. Ibarra and G. F. Goya, Validity of the Néel-Arrhenius Model for Highly Anisotropic CoxFe3−xO4 Nanoparticles, J. Appl. Phys., 2015, 118, 183902 CrossRef
.
- X. Sun, N. Frey Huls, A. Sigdel and S. Sun, Tuning Exchange Bias in Core/Shell FeO/Fe3O4 Nanoparticles, Nano Lett., 2012, 12, 246–251 CrossRef CAS PubMed
.
- J.-H. Lee, J.-t. Jang, J.-s. Choi, S. H. Moon, S.-h. Noh, J.-w. Kim, J.-G. Kim, I.-S. Kim, K. I. Park and J. Cheon, Exchange-Coupled Magnetic Nanoparticles for Efficient Heat Induction, Nat. Nanotechnol., 2011, 6, 418–422 CrossRef CAS PubMed
.
- M. Estrader, A. Lopez-Ortega, S. Estradé, I. V. Golosovsky, G. Salazar-Alvarez, M. Vasilakaki, K. N. Trohidou, M. Varela, D. C. Stanley, M. Sinko, M. J. Pechan, D. J. Keavney, F. Peiró, S. Suriñach, M. D. Baró and J. Nogués, Robust Antiferromagnetic Coupling in Hard–Soft Bi-Magnetic Core/Shell Nanoparticles, Nat. Commun., 2013, 4, 2960 CAS
.
- M. Vasilakaki, K. N. Trohidou and J. Nogues, Enhanced Magnetic Properties in Antiferromagnetic-Core/Ferrimagnetic-Shell Nanoparticles, Sci. Rep., 2015, 5, 9609 CrossRef CAS PubMed
.
- H. Zeng, J. Li, Z. L. Wang, J. P. Liu and S. H. Sun, Bimagnetic Core/Shell Fept/Fe3O4 Nanoparticles, Nano Lett., 2004, 4, 187–190 CrossRef CAS
.
- C. Ostenfeld and S. Mørup, Magnetic Interactions between Nanoparticles of Different Materials, in Hyperfine Interactions (C), 2002, pp. 83–86 Search PubMed
.
- S. Mørup, M. F. Hansen and C. Frandsen, Magnetic Interactions between Nanoparticles, Beilstein J. Nanotechnol., 2010, 1, 182–190 CrossRef PubMed
.
- P. Gambardella, S. Rusponi, M. Veronese, S. S. Dhesi, C. Grazioli, A. Dallmeyer, I. Cabria, R. Zeller, P. H. Dederichs, K. Kern, C. Carbone and H. Brune, Giant Magnetic Anisotropy of Single Cobalt Atoms and Nanoparticles, Science, 2003, 300, 1130–1133 CrossRef CAS PubMed
.
- J. Mohapatra, A. Mitra, D. Bahadur and M. Aslam, Surface controlled synthesis of MFe2O4 (M = Mn, Fe, Co, Ni and Zn) nanoparticles and their magnetic characteristics, CrystEngComm, 2013, 15, 524–532 RSC
.
- J. Riegler, J. A. Wells, P. G. Kyrtatos, A. N. Price, Q. A. Pankhurst and M. F. Lythgoe, Targeted Magnetic Delivery and Tracking of Cells Using a Magnetic Resonance Imaging System, Biomaterials, 2010, 31, 5366–5371 CrossRef CAS PubMed
.
- L. Gutierrez, et al., Synthesis Methods to Prepare Single- and Multi-Core Iron Oxide Nanoparticles for Biomedical Applications, Dalton Trans., 2015, 44, 2943–2952 RSC
.
- J. Li, H. Zeng, S. Sun, J. P. Liu and Z. L. Wang, Analyzing the structure of CoFe–Fe3O4 core–shell nanoparticles by electron imaging and diffraction, J. Phys. Chem. B, 2004, 108, 14005–14008 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |