Small-size and monodispersed red-emitting Pr3+ doped barium molybdate nanocrystals with ultrahigh color purity†
Xuyong Yang*,
Fei Huang,
Zhenwei Huang,
Fan Cao and
Jianhua Zhang*
Key Laboratory of Advanced Display and System Applications, Education of Ministry, Shanghai University, 149 Yanchang Road, P. R. China 200072. E-mail: yangxy@shu.edu.cn; jhzhang@shu.edu.cn
First published on 4th July 2016
Abstract
BaMoO4:Pr3+ phosphors have huge promise as a red emitting component for white LEDs. Here, we report small-size, monodispersed BaMoO4:Pr3+ nanocrystals prepared by an effective liquid–solid–solution (LSS) method. These NCs exhibit narrow-linewidth luminescence emission with ultrahigh color purity for red, which is a perfect combination of both nanoparticles and luminescent properties.
Rare earth doped nanocrystals (NCs) with weak light scattering and easy industrial processing have emerged as a new class of fluorescent materials.1 They offer diverse applications in nanoscale electronics, solid-state lighting, display, solar cells and advanced bioanalysis compared with their macroscopic counterparts.2–9 Currently, the overall conversion efficiency of white light-emitting diodes (LEDs) is only 30%, and to a large extent this low efficiency is attributed to the phosphor. Rare earth doped phosphors such as red Sr(LiAl3N4):Eu2+ (ref. 10) and BaMoO4:Pr3+ (ref. 11) are typically synthesized using solid-state reactions that require annealing temperatures up to above 1000 °C and result in large particle sizes. Multiple scattering of the emission from the micrometer-sized phosphor powders leads to poor beam collimation, substantial backscattering of the emission into the semiconductor chip, and absorption losses in the phosphor itself.12 Reducing the particle size to the nanoscale would essentially eliminate scattering according to particle scattering scales as the square of the particle mass. The synthesis of nanophosphors requires lower temperature methods in which the particles are nucleated, grown, and retained in solution, without aggregation. Some efforts have been done to develop nano-sized phosphors for avoiding the negative effects of particle scattering.13–17 However, it remains a great challenge to prepare small-size and non-aggregated rare earth doped nanophosphors because the synthesis of insulating nanoparticles is hindered by the high lattice energy and low solubility of oxides in both aqueous and nonaqueous solvents.
Here, we reported monodispersed Pr3+ doped BaMoO4 red nanophosphors with an average particle size of 8.7 nm by a simple and effective liquid–solid–solution (LSS) method. Oleic acid (OA) and oleylamine (OLA) are used as a mixed surfactant for cooperatively controlling the crystallization growth of the uniform and non-aggregated NCs. The as-prepared BaMoO4:Pr3+ nanophosphors exhibit a narrow-linewidth red emission with ultrahigh color purity. The effects of the introduction of Na+ ions on the phosphor system have been investigated and the possible mechanism involves the charge compensation.
A typical powder X-ray diffraction (XRD) pattern is presented in Fig. 1a. It can be observed that all of the peaks match those of scheelite-type BaMoO4 (no. JCPDS 29-0193). In the molybdate system with the tetragonal crystal structure, the molybdenum element surrounded by four oxygen atoms is located in the symmetry center of the tetrahedral, as shown in the inset of Fig. 1a. MoO42− has the excellent stability and can effectively adsorb UV/blue light and transfer energy to the rare-earth ions doped in MoO42−. The strong and narrow peaks of XRD indicate a high crystallinity of the as-prepared products, which is beneficial for obtaining high luminescence. Transmission electron microscopy (TEM) was used to further confirm the size and distribution of the BaMoO4:Pr3+ NCs. As shown in Fig. 1b, the lattice fringe with 0.302 nm is identified, which is very close to the inter-plane spacing of (001) plane calculated from XRD data. We can see that the NCs are well dispersed and their size distribution is relatively narrow. Aided by a statistical analysis of a large number of nanocrystals, we determined the average size of nanocrystals to be 8.7 nm, with a standard deviation of ±0.8 nm (Fig. 1c).
 | ||
| Fig. 1 (a) XRD patterns, (b) TEM image and (c) size distribution of the as-prepared BaMoO4:Pr3+ NCs. Inset: the crystal structure of BaMoO4. | ||
To confirm the presence revealed by the structural analysis of Pr3+ species, preliminary X-ray photoelectron spectroscopy (XPS) studies on the as-prepared samples prepared at 160 °C have been undertaken. As shown in Fig. 2, the binding energy of Pr 3d peaking at 923.1 eV and 932.0 eV in the sample are observed. In addition to the successful introduction of Pr3+, the Na+ ions are also found in the XPS spectra of sample (NaOH solution is used to adjust to the reaction pH value), and the energy binding peak of Na 1s is at 1071.6 eV. It has been previously demonstrated that the alkali-metal ions are easily incorporated into the BaMoO4:Pr3+ phosphors in the reaction process and improve the luminescence emission by charge compensation.18–20 In our case, the positive charge defects PrBa+ are produced when a small amount of Pr3+ ions are incorporated into Ba2+ positions (PrBa+), which would lead to the decreased luminescence efficiency. With the introduction of Na+ ions into Ba2+ positions, the obtained negative charge defects NaBa− can play a role in maintaining the charge balance of phosphor system and thus effectively offset the impact of declining luminescence efficiency caused by defects of PrBa+. In addition, alkali metals have the chemical nature of low oxidation states and distinct ionic radii, and therefore the introduction of Na+ ions can modify the local site symmetry of the Pr3+ containing materials, which is in favour of the enhancement for luminescence emission.
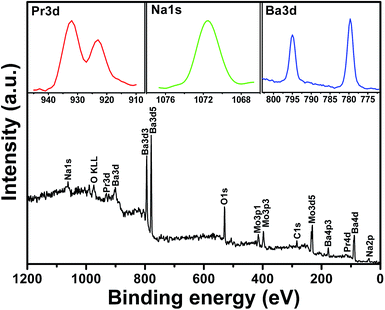 | ||
| Fig. 2 XPS spectra of the as-prepared BaMoO4:Pr3+ NCs. Inset: the binding energy regions for Pr, Na and Ba elements. | ||
The LSS method is adopted to prepare BaMoO4:Pr3+ NCs. The reaction mechanism is depicted in Fig. 3. After an aqueous solution of Ba(NO3)2, Pr(NO3)3, octadecene, oleic acid and oleylamine (OA/OLA as a mixed surfactant21) are added concurrently into a vessel, the barium oleate and praseodymium oleate solution are obtained via a hydrothermal reaction. Next, with the addition of Na2MoO4 and NaOH aqueous solution into the mixture solution in an autoclave, a three-phase system is formed: the solid phase of Ba(OA)2, Pr (OA)3 and residual NaOA (solid), the liquid phase of octadecene, OA and OLA (liquid), and the water solution containing Na+, NO3−, MoO4−, OH− ions (solution). When the mixture is fully stirred, the small liquid drops aqueous solution containing Na2MoO4 surrounded by Ba(OA)2, Pr(OA)3 and NaOA formed microcapsules. At a designated temperature (160 °C) and time (10 h), reactions occurred between M(OA)n (M = Ba2+, Pr3+, or Na+) and Na2MoO4 at the interfaces of the solid–liquid phase, leading to the formation of BaMoO4:Pr3+ NCs. Meanwhile, a mixed layer of OA and OLA was absorbed onto the surface of the obtained NCs, with the alkyl chains facing towards the exterior, thus providing the produced NCs with hydrophobic surfaces.
 | ||
| Fig. 3 Schematic illustration of the reaction mechanism for the synthesis of luminescent BaMoO4:Pr3+ NCs. | ||
The photoluminescence (PL) and PL excitation (PLE) spectra of the BaMoO4:Pr3+ NCs are shown in Fig. 4a. A strong and sharp red emission band centered at 644 nm attributed to the 3P0 → 3F2 transition of the Pr3+ (inset of Fig. 4). The red emission band can be observed under the 449, 473 and 487 nm excitation, which are attributed to 3H4 → 3P2, 3H4 → 3P1 and 3H4 → 3P0 transition, respectively. The 3P0 → 3F2 transition of the Pr3+ emission linewidth (full width at half maxima) is only ∼4 nm, much smaller than those of typical rare earth ions (10–20 nm).22–25 The Commission Internationale del'Eclairage (CIE) chromaticity coordinates of these as-prepared BaMoO4:Pr3+ NCs, SiAlON:Pr3+ red phosphor and the commercial red phosphors of CaS:Eu2+ and Y2O2S:Eu3+ are shown in Fig. 4b. The CIE chromaticity coordinates of Y2O2S:Eu3+, CaS:Eu2+, SiAlON:Pr3+, and BaMoO4:Pr3+ are (0.645, 0.354),26 (0.670, 0.330),27 (0.6851, 0.3148),28 and (0.699, 0.301), respectively. As we observed, the chromaticity point of BaMoO4:Pr3+ NCs is in the deep red region and rather close to the edge of the CIE diagram, indicating its ultrahigh color purity. These red emitting nanophosphors with high color purity can be efficiently excited from 430 nm to 500 nm, which are coupled well with the emission of blue LEDs and thus are potential red candidates as the red emitting phosphors of white LEDs.
In conclusion, the small-size, monodispersed BaMoO4:Pr3+ NCs were synthesized by a simple solution chemistry method. These NCs exhibit excellent luminescence properties with high spectral purity in red and do no requiring annealing process. The co-doping of Na+ ions into the nanophosphor system plays a role in improving the luminescence emission of the as-prepared NCs. The reasons for the emission enhancement involve charge compensation mechanism. Our results suggest that the as-prepared red BaMoO4:Pr3+ nanophosphors are potentially useful as high-performance luminophores in solid-state lighting and displays.
Acknowledgements
The authors would like to thank The Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No. TP2015037) and the key laboratory of advanced display and system applications of ministry of education of China for supporting the research. J. H. Zhang also would like to thank the financial support by Major State Basic Research Development program of China (973; 2015CB655005) and the Science and Technology Commission of Shanghai Municipality (14XD1401800, 14DZ2280900).Notes and references
- H. Terraschke and C. Wickleder, Chem. Rev., 2015, 115, 11352–11378 CrossRef CAS PubMed.
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924–936 CrossRef CAS PubMed.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed.
- R. Lv, P. Yang, F. He, S. Gai, G. Yang and J. Lin, Chem. Mater., 2015, 27, 483–496 CrossRef CAS.
- L. Meng, K. Zhang, K. Pan, Y. Qu and G. Wang, RSC Adv., 2016, 6, 5761–5766 RSC.
- M. Back, E. Trave, R. Marin, N. Mazzucco, D. Cristofori and P. Riello, J. Phys. Chem. C, 2014, 118, 30071–30078 CAS.
- C. Bouzigues, T. Gacoin and A. Alexandrou, ACS Nano, 2011, 5, 8488–8505 CrossRef CAS PubMed.
- R. Adhikari, G. Gyawali, T. H. Kim, T. Sekino and S. W. Lee, Mater. Lett., 2013, 91, 294–297 CrossRef CAS.
- R. Adhikari, B. Joshi, R. Narro-García, E. DelaRosa, T. Sekino and S. W. Lee, Mater. Lett., 2015, 142, 7–10 CrossRef CAS.
- P. Pust, V. Weiler, C. Hecht, A. Tücks, A. S. Wochnik, A.-K. Henß, D. Wiechert, C. Scheu, P. J. Schmidt and W. Schnick, Nat. Mater., 2014, 13, 891–896 CrossRef CAS PubMed.
- X. Yang, Y. Zhou, X. Yu, H. V. Demir and X. W. Sun, J. Mater. Chem., 2011, 21, 9009–9013 RSC.
- M. Nyman, L. E. Shea-Rohwer, J. E. Martin and P. Provencio, Chem. Mater., 2009, 21, 1536–1542 CrossRef CAS.
- P. Ghosh and A.-V. Mudring, Nanoscale, 2016, 8, 8160–8169 RSC.
- S. Y. Kim, J. S. Jeong, K. A. Mkhoyan and H. S. Jang, Nanoscale, 2016, 8, 10049–10058 RSC.
- K. Jayanthi and S. V. Manorama, J. Phys. Chem. C, 2014, 2, 10322–10330 CAS.
- S. Srivastava, A. Mondal, N. K. Sahu, S. K. Behera and B. B. Nayak, RSC Adv., 2015, 5, 11009–11012 RSC.
- P. Kumarab and B. K. Gupta, RSC Adv., 2015, 5, 24729–24736 RSC.
- X. Yang, J. Liu, H. Yang, X. Yu, Y. Guo, Y. Zhou and J. Liu, J. Mater. Chem., 2009, 19, 3771–3774 RSC.
- X. He, M. Guan, Z. Li, T. Shang, N. Lian and Q. Zhou, J. Am. Ceram. Soc., 2011, 94, 2483–2488 CrossRef CAS.
- X. Yang, X. Yu, H. Yang, Y. Guo and Y. Zhou, J. Alloys Compd., 2009, 479, 307–309 CrossRef CAS.
- W. Bu, Z. Chen, F. Chen and J. Shi, J. Phys. Chem. C, 2009, 113, 12176–12185 CAS.
- Q. Dai, M. E. Foley, C. J. Breshike, A. Lita and G. F. Strouse, J. Am. Chem. Soc., 2011, 133, 15475–15486 CrossRef CAS PubMed.
- D. Yue, W. Lu, C. Li, X. Zhang, C. Liu and Z. Wang, Nanoscale, 2014, 6, 2137–2145 RSC.
- T. Ninjbadgar, G. Garnweitner, A. Börger, L. M. Goldenberg, O. V. Sakhno and J. Stumpe, Adv. Funct. Mater., 2009, 19, 1819–1825 CrossRef CAS.
- Y. Liu, J. Zhang, C. Zhang, J. Jiang and H. Jiang, J. Phys. Chem. C, 2016, 120, 2362–2370 CAS.
- L.-Y. Zhou, J.-S. Wei, L.-H. Yi, F.-Z. Gong, J.-L. Huang and W. Wang, Mater. Res. Bull., 2009, 44, 1411–1414 CrossRef CAS.
- H. Takashima, K. Shimada, N. Miura, T. Katsumata, Y. Inaguma, K. Ueda and M. Itoh, Adv. Mater., 2009, 21, 3699–3702 CrossRef CAS.
- T.-C. Liu, B.-M. Cheng, S.-F. Hu and R.-S. Liu, Chem. Mater., 2011, 23, 3698–3705 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: The charge compensation role of doping Na+ ions and the excitation spectrum with UV region of sample. See DOI: 10.1039/c6ra14222b |
| This journal is © The Royal Society of Chemistry 2016 |

