Integration of poly(3-hexylthiophene) conductive stripe patterns with 3D tubular structures for tissue engineering applications†
Yingjuan Sunac,
Hongyan Libc,
Yuan Lin*a,
Li Niub and
Qian Wangad
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Changchun, 130022, P. R. China. E-mail: linyuan@ciac.ac.cn
bState Key Laboratory of Electroanalytical Chemistry, c/o Engineering Laboratory of Modern Analytical Techniques, Changchun Institute of Applied Chemistry, Changchun, 130022, P. R. China
cUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
dDepartment of Chemistry and Biochemistry, University of South Carolina, 631 Sumter Street, Columbia, South Carolina 29208, USA
First published on 25th July 2016
Abstract
3D tubular structures containing spatially distributed conductive stripe patterns of poly(3-hexylthiophene) (P3HT) and polylactic acid (PLA) were generated using a confined evaporative self-assembly (CESA) method on a flexible polyimide (PI) film. These tubular structures could provide contact cues to guide the growth and alignment of pre-osteoblasts and smooth muscle cells. In addition, the spatially electric signals from the conductive stripes could regulate the proliferation and osteogenic differentiation of pre-osteoblasts. This simple and effective strategy has the potential to mimic tubular tissues and has great promise in bone, cardiac and neural tissue engineering applications.
1. Introduction
In many organs and tissues, cells are organized as tubular structures with curved shapes to support specific properties and biological functions.1–4 In order to better mimic such three-dimensional (3D) tubular tissues in vivo, a variety of techniques have been developed, including electrospun techniques,5 thin film rolling-up induced by the internal stress of materials6–10 or by scrolling with an external force,11,12 and flow-assembly in capillary tubes, etc.13,14 Meanwhile, cellular alignment plays a crucial role in the microarchitecture of many human tissues, dictating their biological and mechanical functions.15 Cells respond to topography over spatial dimensions of nano to micrometer and anisotropic structures seem to be more conducive to induce cells alignment.16 It was reported that pre-aligned myoblasts would better mimic the physiologic process of muscle formation and enhance structural organization.17 On the other hand, it is known that electric stimulation (ES) could modulate protein adsorption, cell attachment, adhesion, migration, differentiation and other cellular behaviours.18,19 For example, Xia and coworkers utilized electrospun method to fabricate conducting polypyrrole (PPy) core-sheath tubular structures to induce neurites outgrowth, while they found the maximum length of neurites increased under electric stimulation.20 Similarly, PPy has been galvanostatically polymerized on the wet-spun fibers onto the mylar, after rolling-up into tubular shape, electric stimulation allows axon growth and Schwann cell migration.21 It is well known that, at the level of the muscle, axons beneath the stimulating electrodes are located widely distributed.22 The spatial distributed stimulation might have the ability to maximize selective activation of a cell while minimizing activation of neighboring cells.23However, controlling the locations of conductive polymers (CPs) in the tubular scaffolds to achieve spatial distribution of conductive pattern is still a challenge. Current reports only loaded the CPs on the fibers template or covered the CPs film on the substrate surface, thus these methods could not precisely regulate the locations of the electric signals in the tubular scaffolds. CPs were reported that they could generate electric signals by electron transfer between different polymer chains, where electric stimulation through such materials would be predominantly localized to the surrounding CPs, thus allowing for the spatial control of electric stimulation.18,24 Our group has reported that parallel poly(3-hexylthiophene) (P3HT) stripes could be fabricated on the 2D substrates by confined evaporative self-assembly (CESA). The distribution of the conductive signals could be precisely controlled by tuning the locations of P3HT stripes. In particular, the P3HT backbone orientation in individual stripe could be controlled at the molecular level, which resulted in a much higher electrical conductivity than normal P3HT aggregates.25,26
Herein, we present a facile way to fabricate stripe patterns consisting of conductive polymer (P3HT) and biodegradable polymer (polylactic acid, PLA) on flexible polyimide (PI) films27 using CESA method (Scheme 1).28–34 In order to mimic cells growth and alignment in different tubular tissues in vivo, the pre-patterned pre-osteoblasts cells on PI films were rolled-up along y-axis to make 3D tubular scaffolds while smooth muscle cells (SMCs) remained aligned along x-axis within the tubes. These structures could provide the contact guidance for cells alignment. In addition, the providing spatial electric signals have been employed to further modulate cellular behaviours.
2. Experimental
2.1. Materials
Regioregular P3HT (Mw = 45 kDa, PDI = 1.8, RR = 98%) was purchased from Rieke Metals Inc. and PLA (Mw = 100 kDa, PDI = 1.5) was purchased from Sigma Aldrich. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), alizarin red S (ARS), β-glycerolphosphate, L-ascorbic acid 2-phosphate, dexamethasone were all purchased from Sigma Aldrich. Polyimide (PI) films were purchased from DuPont Co. The quartz cylindrical lenses (L = 12 mm, W = 10 mm, R = 12.92 mm), glass slides (L = 12 mm, W = 12 mm) were cleaned with Piranha solution (H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 = 7
H2O2 = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) for 2 h to remove the stains on surface (Caution! Piranha solution reacts violently with organic materials), then washed thoroughly with deionized water for three times and dried under nitrogen flow. PI films were washed with ethanol and deionized water.
3) for 2 h to remove the stains on surface (Caution! Piranha solution reacts violently with organic materials), then washed thoroughly with deionized water for three times and dried under nitrogen flow. PI films were washed with ethanol and deionized water.
2.2. Sample fabrication
Cylindrical lenses were situated on a flat substrate (PI film were pasted on glass slides) to construct the cylinder-on-flat geometry. P3HT blending with different molar ratios of PLA in CF solution (30 μL) was trapped within the gap between the cylinder and substrate due to the capillary force. After evaporation (at room temperature 23 ± 2 °C and 20–30% in humidity), 12 mm in length and 10 mm in width patterns were produced on PI films. The temperature and humidity were monitored by temperature-hygrometer (Hygrometer Testo 608-H1, Germany). Spin coating P3HT&PLA solutions on PI films were characterized on a Homogenizer (KW-4A, China) at the speed of 1000 rpm for 1 min. Au electrodes were fabricated from a gold sheet through hot splash vapor deposition (ZZS700-2/G, Nanguang, China) method on the patterns via a template.2.3. Cell experiments
Pre-osteoblasts (MC 3T3-E1 cells) and smooth muscle cells (A7r5 cells) were cultured in Dulbecco's modified Eagle's medium (DMEM) with high glucose containing 10% fetal bovine serum (FBS) from Gibco Inc., penicillin (100 U mL−1) and streptomycin (100 μg mL−1) at 37 °C in a 5% CO2 humidified atmosphere. Cells were washed twice with DMEM. Later, living cells were stained with FDA (2.5 μg mL−1) for 5 min before observation. Actin fibres were stained with phalloidin-FITC (1 μg mL−1) and the nuclei were stained by DAPI (1 μg mL−1).2.4. Electric stimulation of cells
MC 3T3-E1 cells (105 cells per well, 6-well plate) were seeded on the planar patterns for 12 h prior to the induction of cells differentiation by replacing the original medium with osteogenic medium consisting of DMEM supplemented with 10% FBS, penicillin (100 U mL−1), sodium β-glycerolphosphate (10 mM), streptomycin (100 μg mL−1), L-ascorbic acid 2-phosphate (50 μg mL−1) and dexamethasone (10−8 M). Media were replenished every 2 days. And A7r5 cells (105 cells per well, 6 well plate) were cultured as the same density in the original medium. Gold electrodes were sprayed on the self-assembled stripe patterns using a template with the distance of 1 cm, which was illustrated in Fig. S1.† Then the cell-patterned PI films were rolled up into a 3 mm diameter tube. After the cells adhesion and alignment for 12 h, the electrical signal was added directly on the substrates through two platinum electrodes (1 mm in diameter) with the distance of 1 cm. Electric stimulation used here is in order to enhance the proliferation and differentiation of cells. The electric stimulation was carried out on the Signal Generator (Rigol DG1022U DDS) and the signals were checked on the wave inspector (Rigol DS1022C digital oscilloscope). Square wave, frequency of 100 Hz, 50% duty cycle, and electric potential of 500 mV was adopted on the MC 3T3-E1 cells and on the A7r5 cells in the experiments. The samples were respectively stimulated for 1 h every day.2.5. MTT assay of cells viability
After 7 d and 14 d of electrical stimulation, the MC 3T3-E1 cells culture medium were replaced with 1.8 mL DMEM and 200 μL MTT (5 mg mL−1 in PBS) and the scaffolds were incubated for another 4 h at 37 °C. Then the solutions were removed and 1 mL dimethyl sulfoxide (DMSO) was added into them. The scaffolds were gently agitated until the formazan precipitate was dissolved. The same way was applied on the SMCs to detect the proliferation. OD values were measured using a spectrophotometer at 490 nm. MTT assay was characterized on a Tecan infinite 200 spectrophotometer (TecanGroup Ltd).2.6. Alizarin red staining and quantification
MC 3T3-E1 cells with calcium deposits due to bone nodule formation were stained with alizarin red. The staining was performed according to reference.35 Briefly, the medium was aspirated out from each well, and cells were fixed with 4% paraformaldehyde for 30 min at room temperature. Then the cells were rinsed twice with ultrapure water followed by addition of 2 mL of 0.2% alizarin red solution for each well and incubated for 1 h. Finally, the unstained alizarin red was washed with ultrapure water, and the substrates were visualized under a microscope. Alizarin red quantification was done using a previously reported procedure.362.7. Characterization
Optical microscopy measurements were carried out using a Carl Zeiss A1m microscope with a charge-coupled device camera. AFM characterization was obtained on an Agilent 5500 AFM by tapping mode in an ambient atmosphere. CLSM images were characterized by a LSM 700 with a Zeiss microscopy. UV-Vis was measured on a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific). The SEM images were obtained from the Phenom scanning electron microscope Pro-x PW-100-011 (part nr), 800-07333 (model nr) system (PHENON WORLD) at a 10 kV voltage. I–V curve was measured on a Keithley-2400 sourcemeter with the distance between the two Au electrodes was 100 μm. Raman spectra were obtained with LabRam HR800 spectrometer (Horiba Jobin Yvon) equipped with an Olympus BX41 microscope in the backscattering geometry. A 632.8 nm He–Ne laser was focused on the sample with a 50× objective lens. Stripes were aligned along the z direction (parallel to the long-axis direction of the stripes) and polarized spectra were recorded in the order: zz, xx, ratios were obtained by measuring the intensity ten different points in one stripe at different positions. We define z direction as that parallel to the long axis of the stripe and x perpendicular to the stripe long axis in the sample plane. For the measurements with polarized light, we use two configurations, zz and xx, using the notation “incident polarization analyzed polarization”.3. Results and discussion
3.1. Preparation of 3D conductive tubular scaffolds of pre-osteoblasts
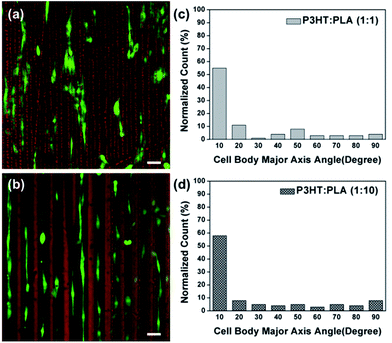
Concentration of P3HT solution was fixed as 0.1 mg mL−1 in chloroform. With the molar ratio of PLA in the blending solution increased (ranging from 0.1 mg mL−1, 0.5 mg mL−1 to 1 mg mL−1), regular stripes were obtained and P3HT molecules distributed evenly in each stripe (Fig. 1a–c). Surface morphology of single stripe was characterized by AFM (Fig. 1d–f). The distance between the adjacent stripes (from 20 μm to 45 μm) and the height of the stripes in outer regions increased (from ∼100 nm to ∼250 nm), which were shown in Fig. 1g. Chain orientations of P3HT molecules within an individual stripe could be monitored by confocal polarized Raman spectroscopy.25,26,28 With the percentage of PLA increased in the blending solution, the chain orientation of P3HT molecules in single stripe changed slightly (Fig. 1h), indicating that the addition of PLA had almost no effects on the ordered arrangement of P3HT molecules in the stripe. Moreover, the stripe patterns were still conductive (Table S1, in ESI†) after blending with PLA. In addition, the patterns were very stable which could last at least 14 days in the cell culture medium (Fig. S2†). Such good stability permits the following long-term cell-culturing studies.37 For better mimicking of cells growth and alignment in different tubular tissues in vivo, the stripe patterns on PI films were rolled-up along y-axis or x-axis to construct different orientated 3D conductive tubular structures, which was illustrated in Scheme 1.Therefore, we first cultured the pre-osteoblasts38 (MC 3T3-E1 cells) on the conductive stripe patterns prior to the formation of tubular scaffolds. The P3HT&PLA thin film prepared via spin coating was used as control. The alignment was quantified by measuring the angle between the cell nuclei and long axis of the stripe (n > 200) from a series of fluorescence images, where 0° denoted parallel alignment to the axis of the stripe and 90° represented perpendicular alignment. About 80% of cells nuclei were aligned within 20° of the long axis of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA (1
PLA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) stripes (Fig. 2a and d). They were separated in space and fell into the spacing between the adjacent stripes tightly and became highly elongated in aligning with the direction of the stripes as shown in Fig. 2b and c.
5) stripes (Fig. 2a and d). They were separated in space and fell into the spacing between the adjacent stripes tightly and became highly elongated in aligning with the direction of the stripes as shown in Fig. 2b and c.
Similar phenomena of the cells could be observed when they were cultured on the P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA (1
PLA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) patterned stripes (Fig. 3b and d). For the P3HT
10) patterned stripes (Fig. 3b and d). For the P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA (1
PLA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) stripes, the narrow spacing induced most of the cells spreading across the stripes (Fig. 3a and c), but still parallel to the long axis of the stripes. As comparison, well adhered cells randomly spread and oriented freely in all directions on the P3HT&PLA thin films fabricated by spin coating (Fig. 2e–h). It was reported that the width and height are two key factors to influence the cells alignment on the grooves topography.39,40 Though the heights of these stripe patterns here are nano-grade, different response was observed mainly due to the contact guidance of the topography.
1) stripes, the narrow spacing induced most of the cells spreading across the stripes (Fig. 3a and c), but still parallel to the long axis of the stripes. As comparison, well adhered cells randomly spread and oriented freely in all directions on the P3HT&PLA thin films fabricated by spin coating (Fig. 2e–h). It was reported that the width and height are two key factors to influence the cells alignment on the grooves topography.39,40 Though the heights of these stripe patterns here are nano-grade, different response was observed mainly due to the contact guidance of the topography.
Subsequently, tubular scaffolds were formed by rolling-up the patterned PI films. Considering that many medium vessels in osteonal are ranged from 2 to 10 mm in diameter,37 we first made 3 mm tubes. The P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PLA (1
PLA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) samples were chosen to optimize the cells alignment. MC 3T3-E1 cells were allowed to pre-adhere on the surfaces for 12 h, then the patterned PI films together with cells were rolled-up along y-axis (Scheme 1). To better characterize the 3D structure of the cell loading tubes, 3D reconstruction of z-stack fluorescence images of cells in the tubes were shown in Fig. 4a and b. From different views, we could observe the cell sheet (green) and the stripe patterns (red), which was more beneficial to the interactions between cells and materials and the following electrical stimulation of cells towards the conductive stripe patterns. Similarly, tubes with different diameters (Fig. 4c), i.e. ranging from 1.5 mm to 4 mm (the sizes of medium blood vessels), were prepared and analysed, and all systems showed good alignment of the pre-osteoblasts (Fig. 4d). Further, multi-layered tubes could also be constructed using the same method (Fig. S3a†). Pre-osteoblasts still survived on the first (Fig. S3b†) and the second layer (Fig. S3c†), but the alignment was not completely maintained. Due to the lack of transport of nutrients, there were almost no living cells on the third layer (Fig. S3d†).
5) samples were chosen to optimize the cells alignment. MC 3T3-E1 cells were allowed to pre-adhere on the surfaces for 12 h, then the patterned PI films together with cells were rolled-up along y-axis (Scheme 1). To better characterize the 3D structure of the cell loading tubes, 3D reconstruction of z-stack fluorescence images of cells in the tubes were shown in Fig. 4a and b. From different views, we could observe the cell sheet (green) and the stripe patterns (red), which was more beneficial to the interactions between cells and materials and the following electrical stimulation of cells towards the conductive stripe patterns. Similarly, tubes with different diameters (Fig. 4c), i.e. ranging from 1.5 mm to 4 mm (the sizes of medium blood vessels), were prepared and analysed, and all systems showed good alignment of the pre-osteoblasts (Fig. 4d). Further, multi-layered tubes could also be constructed using the same method (Fig. S3a†). Pre-osteoblasts still survived on the first (Fig. S3b†) and the second layer (Fig. S3c†), but the alignment was not completely maintained. Due to the lack of transport of nutrients, there were almost no living cells on the third layer (Fig. S3d†).
3.2. Electric stimulation of pre-osteoblasts in the 3D conductive tubular scaffolds
Electric stimulation (ES) was performed after the cells adhesion and alignment for 12 h on the conductive stripe patterns, which is for the purpose of enhancing the proliferation and differentiation of cells, resulted in significantly greater MTT values in stimulated groups than the non-stimulated ones and the control spin coating films (Fig. 5a), thus demonstrating that cells proliferation were promoted by the spatial distributed electrical stimulation. It provided the prediction that spatial stimulation signals maximized selective activation of a cell while minimizing activation of neighboring cells,22,23 which was more helpful in enhancing the proliferation. Intracellular free calcium concentration is one of the functional variables to measure osteogenic effects of the electrical factor on cell differentiation.41 More calcium depositions occurred on the stimulated groups (Fig. S4a, c, e and g†) than the non-stimulated groups (Fig. S4b, d, f and h†). The calcium deposition values in stimulated groups were almost 3-fold higher than the non-stimulated ones which were measured by alizarin red staining (Fig. 5b). In addition, more calcium depositions were observed after the cells were stimulated for 14 d (Fig. S4c, d, g and h†). It has been reported that cell density are related to differentiation and high density is beneficial to osteoblasts mineralization.41 That might be why we observed more cells aggregation on the spin coating films than the discontinuous stripes because of the gaps between the adjacent stripes. These results demonstrated that ES could effectively promote the proliferation and osteogenic differentiation of pre-osteoblasts through the spatial electric signals from the conductive stripe patterns.3.3. Preparation of 3D conductive tubular scaffolds of smooth muscle cells
To further demonstrate the versatile utility of our method in tissue engineering applications, the controlled culturing of smooth muscle cells (SMCs) using the aforementioned tubular scaffolds were explored. SMCs are normally circumferentially aligned inside the tunica media in a native wall of blood vessel in vivo to achieve its natural function, therefore we rolled-up the PI thin films along the x-axis as shown in Scheme 1 to ensure the P3HT stripes circumferentially aligned inside the tube.42,43 SMCs were pre-cultured on these two-dimensional scaffolds, showing a less-spread, elongated bipolar morphology (Fig. 6a and b). The cells were oriented preferentially parallel to the long axis of the stripes with about 75% of cells nuclei aligned within 20° of the stripes (Fig. 6g). In contrast, cells exhibited random orientation, spread-out morphology and well-formed cell–cell adhesions when they were cultured on control spin coating films (Fig. 6c, d and h). Pre-patterned SMCs on the PI films could be rolled-up parallel to the direction of x-axis (perpendicular to the long axis of stripes), and a 3D reconstruction of z-stack fluorescence images of SMCs in the tube were shown in Fig. 6e and f.The cell sheet (green) was above the stripe patterns (red), which was similar to the tunica of blood vessel wall in vivo. Upon ES, similar results were obtained from the proliferation statistics of SMCs (Fig. 6i), stimulated groups had higher MTT values than non-stimulated ones. After stimulation for 3 days, the MTT values in stimulated groups were nearly 1.3-fold than that in non-stimulated groups. These results confirmed that ES performed on the spatial conductive patterns could indeed promote the proliferation of SMCs. We speculate that the ES increased Ca2+ concentration by inducing the cells membrane depolarisation, which subsequently augmented the cells proliferation.42,44
4. Conclusions
In summary, we have successfully fabricated conductive P3HT&PLA stripe patterns with a facile and simple CESA method on flexible PI films. Furthermore, the pre-patterned cells could be rolled-up either along y-axis or x-axis to make 3D tubular cell complexes. The cells could still maintain the alignment guided by the polymer stripes. In addition, electric stimulation could be applied via the conductive polymer stripes in order to stimulate the proliferation of both pre-osteoblasts and smooth muscle cells. Finally, the spatial distributed electric stimulation could also accelerate the osteogenic differentiation of the pre-osteoblasts effectively. Therefore, this strategy allows us to combine the contact guidance cues with the spatial electric signals in 3D tubular scaffolds, and provides a promising approach for potential applications in vascular, muscular and neural tissue engineering.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21374119 and 21429401). YL and QW are also grateful of the support from the State Key Laboratory of Polymer Physics and Chemistry of the Chinese Academy of Sciences. YL also thanks the support from the State Key Laboratory of Precision Measuring Technology and Instrument of Tianjin University.Notes and references
- L. Robert, Pathol. Biol., 2001, 49, 279–283 CrossRef CAS PubMed.
- E. Eisenbarth, P. Linez, V. Biehl, D. Velten, J. Breme and H. F. Hildebrand, Biomol. Eng., 2002, 19, 233–237 CrossRef CAS PubMed.
- S. Sun, I. Titushkin and M. Cho, Bioelectrochemistry, 2006, 69, 133–141 CrossRef CAS PubMed.
- R. Arayanarakool, A. K. Meyer, L. Helbig, S. Sanchezbcd and O. G. Schmidt, Lab Chip, 2015, 15, 2981–2989 RSC.
- D. Zhang and J. Chang, Nano Lett., 2008, 8, 3283–3287 CrossRef CAS PubMed.
- B. Yuan, Y. Jin, Y. Sun, D. Wang, J. Sun, Z. Wang, W. Zhang and X. Jiang, Adv. Mater., 2012, 24, 890–896 CrossRef CAS PubMed.
- Y. Li, B. Yuan, H. Ji, D. Han, S. Chen, F. Tian and X. Jiang, Angew. Chem., Int. Ed., 2007, 46, 1094–1096 CrossRef CAS PubMed.
- M. Yu, Y. Huang, J. Ballweg, H. Shin, M. Huang, D. E. Savage, M. G. Lagally, E. W. Dent, R. H. Blick and J. C. Williams, ACS Nano, 2011, 5, 2447–2457 CrossRef CAS PubMed.
- P. Gong, W. Zheng, Z. Huang, W. Zhang, D. Xiao and X. Jiang, Adv. Funct. Mater., 2013, 23, 42–46 CrossRef CAS.
- Y. Jin, N. Wang, B. Yuan, J. Sun, M. Li, W. Zheng, W. Zhang and X. Jiang, Small, 2013, 9, 2410–2414 CrossRef CAS PubMed.
- S. Rayatpisheh, D. E. Heath, A. Shakouri, P. Rujitanaroj, S. Y. Chew and M. B. Chan-Park, Biomaterials, 2014, 35, 2713–2719 CrossRef CAS PubMed.
- B. J. Papenburg, J. Liu, G. A. Higuera, A. Barradas, J. Boer, C. A. Blitterswijk, M. Wessling and D. Stamatialis, Biomaterials, 2009, 30, 6228–6239 CrossRef CAS PubMed.
- Y. Wu, S. Feng, X. Zan, Y. Lin and Q. Wang, Biomacromolecules, 2015, 16, 3466–3472 CrossRef CAS PubMed.
- X. Zan, S. Feng, E. Balizan, Y. Lin and Q. Wang, ACS Nano, 2013, 7, 8385–8396 CrossRef CAS PubMed.
- H. Aubin, J. Nichol, C. Hutson, H. Boe, A. Sieminski, D. Cropek, P. Akhyari and A. Khademhosseini, Biomaterials, 2010, 31, 6941–6951 CrossRef CAS PubMed.
- L. Cai, L. Zhang, J. Dong and S. Wang, Langmuir, 2012, 28, 12557–12568 CrossRef CAS PubMed.
- M. T. Lam, Y. Huang, R. K. Birla and S. Takayama, Biomaterials, 2009, 30, 1150–1155 CrossRef CAS PubMed.
- C. E. Schmidt, V. R. Shastri, J. P. Vacanti and R. Langer, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 8948–8953 CrossRef CAS.
- A. Kotwal and C. E. Schmidt, Biomaterials, 2001, 22, 1055–1064 CrossRef CAS PubMed.
- J. Xie, M. R. MacEwan, S. M. Willerth, X. Li, D. W. Moran, S. E. Sakiyama-Elbert and Y. Xia, Adv. Funct. Mater., 2009, 19, 2312–2318 CrossRef CAS PubMed.
- A. F. Quigley, J. M. Razal, B. C. Thompson, S. E. Moulton, M. Kita, E. L. Kennedy, G. M. Clark, G. G. Wallace and R. M. I. Kapsa, Adv. Mater., 2009, 21, 4393–4397 CrossRef CAS PubMed.
- J. Rodriguez-Falces, N. A. Maffiuletti and N. Place, Muscle Nerve, 2013, 48, 752–761 CrossRef PubMed.
- L. H. Jepson, P. Hottowy, K. Mathieson, D. E. Gunning, W. Dąbrowski, A. M. Litke and E. J. Chichilnisky, J. Neurosci., 2014, 34, 4871–4881 CrossRef CAS PubMed.
- L. Ghasemi-Mobarakeh, M. P. Prabhakaran, M. Morshed, M. H. Nasr-Esfahani, H. Baharvand, S. Kiani, S. Al-Deyab and S. Ramakrishna, J. Tissue Eng. Regener. Med., 2011, 5, E17–E35 CrossRef CAS PubMed.
- M. Baibarac, M. Lapkowski, A. Pron, S. Lefrant and I. Baltog, J. Raman Spectrosc., 1998, 29, 825–832 CrossRef CAS.
- G. Xiao, Y. Sun, W. Xu, Y. Lin, Z. Su and Q. Wang, RSC Adv., 2015, 5, 76472–76475 RSC.
- M. Voisin, M. Ball, C. O'Connell and R. Sherlock, Nanomedicine, 2010, 6, 35–43 CAS.
- G. Xiao, Y. Guo, Y. Lin, X. Ma, Z. Su and Q. Wang, Phys. Chem. Chem. Phys., 2012, 14, 16286–16293 RSC.
- W. Han, M. He, M. Byun, B. Li and Z. Lin, Angew. Chem., Int. Ed., 2013, 52, 2564–2568 CrossRef CAS PubMed.
- B. Li, W. Han, B. Jiang and Z. Lin, ACS Nano, 2014, 8, 2936–2942 CrossRef CAS PubMed.
- B. Li, C. Zhang, B. Jiang, W. Han and Z. Lin, Angew. Chem., Int. Ed., 2015, 54, 4250–4254 CrossRef CAS PubMed.
- M. Byun, W. Han, B. Li, X. Xin and Z. Lin, Angew. Chem., Int. Ed., 2013, 52, 1122–1127 CrossRef CAS PubMed.
- M. Byun, R. L. Laskowski, M. He, F. Qiu, M. Jeffries-EL and Z. Lin, Soft Matter, 2009, 5, 1583–1586 RSC.
- W. Han, B. Li and Z. Lin, ACS Nano, 2013, 7, 6079–6085 CrossRef CAS PubMed.
- P. Sitasuwan, L. A. Lee, B. Peng, E. N. Davis, Y. Lin and Q. Wang, Integr. Biol., 2012, 4, 651–660 RSC.
- C. A. Gregory, W. G. Gunn, A. Peister and D. J. Prockop, Anal. Biochem., 2004, 329, 77–84 CrossRef CAS PubMed.
- X. Chen, A. Ergun, H. Gevgilili, S. Ozkan, D. M. Kalyon and H. Wang, Biomaterials, 2013, 34, 8203–8212 CrossRef CAS PubMed.
- M. A. K. Liebschner, Biomaterials, 2004, 25, 1697–1714 CrossRef CAS PubMed.
- S. Ankam, M. Suryana, L. Y. Chan, A. A. K. Moe, B. K. K. Teo, J. B. K. Law, M. P. Sheetz, H. Y. Low and E. K. F. Yim, Acta Biomater., 2013, 9, 4535–4545 CrossRef CAS PubMed.
- A. A. K. Moe, M. Suryana, G. Marcy, S. K. Lim, S. Ankam, J. Z. W. Goh, J. Jin, B. K. K. Teo, J. B. K. Law, H. Y. Low, E. L. K. Goh, M. P. Sheetz and E. K. F. Yim, Small, 2012, 8, 3050–3061 CrossRef CAS PubMed.
- H. Cui, Y. Liu, M. Deng, X. Pang, P. Zhang, X. Wang, X. Chen and Y. Wei, Biomacromolecules, 2012, 13, 2881–2889 CrossRef CAS PubMed.
- M. Zhu, Z. Wang, J. Zhang, L. Wang, X. Yang, J. Chen, G. Fan, S. Ji, C. Xing, K. Wang, Q. Zhao, Y. Zhu, D. Kong and L. Wang, Biomaterials, 2015, 61, 85–94 CrossRef CAS PubMed.
- A. S. Rowlands and J. J. Cooper-White, Biomaterials, 2008, 29, 4510–4520 CrossRef CAS PubMed.
- P. Du, S. Li, G. O'Grady, L. K. Cheng, A. J. Pullan and J. D. Z. Chen, Am. J. Physiol.: Gastrointest. Liver Physiol., 2009, 297, G672–G680 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Stability and conductivity of the stripe patterns, fluorescence images of cells in multi-layered PI tubes, optical images of ARS staining. See DOI: 10.1039/c6ra14109a |
| This journal is © The Royal Society of Chemistry 2016 |