Synthesis and characterization of photodynamic activity of an iodinated Chlorin p6 copper complex†
Paromita Sarbadhikarya,
Alok Dube*ab and
Pradeep Kumar Guptaab
aHomi Bhabha National Institute, Raja Ramanna Centre for Advanced Technology, Indore 452013, India
bLaser Biomedical Application and Instrumentation Division, Raja Ramanna Centre for Advanced Technology, Indore 452013, India. E-mail: okdube@rrcat.gov.in; Tel: +91 731 2488437
First published on 1st August 2016
Abstract
We report the synthesis of a new iodinated Chlorin p6 copper complex (ICp6–Cu) and its efficacy for photodynamic treatment (PDT) of cancer cells. The metal complex is obtained by reacting Chlorin p6 (Cp6) with copper iodide (CuI). The complex formation results in a shift in the Q absorption band of Cp6 from 663 nm to 634 nm and X-ray fluorescence of the complex showed the presence of both copper and iodine. FTIR and EPR spectroscopy suggests that the copper is attached to Cp6 at the two adjacent carboxylic groups. Studies on the photochemical generation of singlet oxygen (1O2) and other reactive oxygen species (ROS) using fluorescence probes revealed that ICp6–Cu acts predominantly through the type I process. PDT of oral cancer cells with ICp6–Cu (10 μM, 3 h) and red light (630 ± 20 nm, ∼12 J cm−2) led to ∼90% phototoxicity. Furthermore, in contrast to Cp6, the phototoxicity induced by ICp6–Cu is not significantly affected under hypoxic conditions.
Introduction
Photodynamic therapy (PDT) of cancer utilizes a photosensitive drug referred to as a photosensitizer (PS) and irradiation with light of appropriate wavelengths to induce tumor damage via generation of ROS.1,2 The absorption of light by the PS results in its excitation to a singlet excited state (1PS*) which undergoes inter system crossing (ISC), to form a relatively long lived excited-triplet state (3PS). The 3PS can undergo two types of reactions defined as type I and type II process. In type I process, the 3PS reacts with surrounding substrates involving transfer of hydrogen atom or electron to result in formation of ion radicals or ROS such as superoxide (O2˙−) and hydroxyl radicals (˙OH). In type II process, 3PS reacts directly with 3O2 through transfer of energy to result in formation of 1O2.3 It is generally accepted that the 1O2 is the major species responsible for PDT efficacy of most PSs.3 Although, PSs which acts via type I process have also been considered useful for PDT.4Currently, PSs such as Photofrin, ALA, Foscan and Verteporfin have been clinically approved and some more PSs are under clinical trials.1 For improving, the tumor selectivity and efficacy of PDT, PSs complexed to metal ions have been actively explored.5 The insertion of metal in PS provides two significant advantages. First, the metal through heavy atom effect can enhance triplet state yield of the PS and second because of cationic charge the PS can accumulate more efficiently in cancer cells.5,6 In addition, metal based PS have received considerable attention for potential use as multimodal agents in tumor imaging and therapy such as magnetic resonance imaging (MRI), positron emission tomography (PET) imaging,7,8 X-ray photon activation therapy (PAT),9–11 and radiotherapy of cancer.12
Our earlier studies in hamster cheek pouch model have shown that Cp6, a water soluble chlorophyll derivative is a promising PS for PDT of tumors.13,14 Cp6 is anionic due to the presence of carboxylic groups in the molecule. For further developing its use as a multimodal agent for PDT and PAT, we have explored the possibility to obtain its metal complex by chelation of metal ion at the carboxylic groups. We choose CuI to prepare complex with Cp6 because of several considerations; copper complexes are potential agents for chemo and radiotherapy of cancer,15,16 iodinated compounds are useful for PAT and contrast imaging,17,18 the radioisotopes of both copper and iodine can provide modality for PET and single-photon emission computed tomography (SPECT) imaging of tumor,19,20 and both copper and iodine are considered to be relatively well tolerated metabolically because these are required as trace elements.21 In this report, we describe the synthesis of an iodinated–Chlorin p6 copper complex (ICp6–Cu) and its characterization as a PS. Unlike the tetrapyrrole copper complex reported in literatures which are metallated at the pyrrole NH, the copper in ICp6–Cu is attached to the carboxylic groups. Studies on 1O2 and ROS generation showed that ICp6–Cu predominantly acts through type I photochemical pathway and as compared to Cp6, it is more effective for PDT of cancer cells under hypoxic condition.
Results and discussion
Formation of complex of Cp6 with CuI
The changes in the absorption spectrum of Cp6 upon addition of different concentrations of CuI are shown in Fig. 1a. With the increase in the concentration of CuI, the absorbance of Q band at 663 nm gradually decreases with concomitant appearance of a new band at 634 nm. This is also accompanied by a decrease in absorbance at the Soret band (400 nm) and the minor Q band (500 nm). Beyond 5 μM concentration of CuI which is equi-molar to Cp6, no further changes were observed. This indicates that the complex formation between Cp6 and CuI is completed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Complex thus formed displays a Soret band with sharp peak at 408 nm with a shoulder at 380 nm, a minor Qx band at 500 nm and the major Qy band at 634 nm. The molar extinction coefficient (ε) of the complex at Qy band is almost same (24
1 stoichiometry. Complex thus formed displays a Soret band with sharp peak at 408 nm with a shoulder at 380 nm, a minor Qx band at 500 nm and the major Qy band at 634 nm. The molar extinction coefficient (ε) of the complex at Qy band is almost same (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) as that for Cp6, whereas at the Soret band the ε is ∼30% lower (63
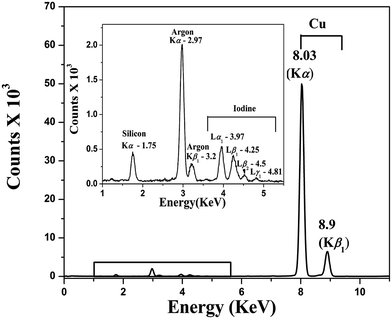
000 M−1 cm−1) as that for Cp6, whereas at the Soret band the ε is ∼30% lower (63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1). In Fig. 1b the fluorescence spectra of Cp6 and ICp6–Cu complex are shown. As compared to Cp6, the fluorescence yield for ICp6–Cu is reduced by a factor of ∼16. This is because of insertion of paramagnetic copper in Cp6 and is consistent with the quenching of fluorescence reported for copper complex of some chlorophyll derivatives.22 X-Ray fluorescence (XRF) spectrum of the complex (Fig. 2) shows the characteristic X-ray emission lines for copper at 8.03 keV (Kα) and 8.9 keV (Kβ1) and for iodine at 3.97, 4.25, 4.5, 4.81 keV (Lα1, Lβ1, Lβ2, Lγ1). This also confirms the presence of both copper and iodine in the complex.
000 M−1 cm−1). In Fig. 1b the fluorescence spectra of Cp6 and ICp6–Cu complex are shown. As compared to Cp6, the fluorescence yield for ICp6–Cu is reduced by a factor of ∼16. This is because of insertion of paramagnetic copper in Cp6 and is consistent with the quenching of fluorescence reported for copper complex of some chlorophyll derivatives.22 X-Ray fluorescence (XRF) spectrum of the complex (Fig. 2) shows the characteristic X-ray emission lines for copper at 8.03 keV (Kα) and 8.9 keV (Kβ1) and for iodine at 3.97, 4.25, 4.5, 4.81 keV (Lα1, Lβ1, Lβ2, Lγ1). This also confirms the presence of both copper and iodine in the complex.
Position of copper and iodine in the complex
Generally, metallation of tetrapyrrole compounds leads to insertion of metal atom in the centre of the tetrapyrrole ring by substitution with two hydrogen at the pyrrole NH. However for tetrapyrrolic compound containing carboxylic groups, it has been reported that the insertion of metal ion at pyrrole NH is not favoured due to preferential binding of metal ion at the carboxylic groups through coordination.23 Cp6 contains three carboxylic groups, one is as propionate side chain at C17 position and remaining two are adjacently attached at C13 and C15 position.To confirm the coordination site of copper in ICp6–Cu, its Electron Paramagnetic Resonance (EPR) spectrum was measured at 100 K in methanol (Fig. 3). The spectrum is anisotropic with characteristic intense absorption in high field region and well resolved hyperfine splitting in low field region. It is important to mention that the EPR spectra of copper complexes of porphyrin and chlorophyll derivatives show characteristic multiple super hyperfine splitting in g⊥ region due to interaction of unpaired electron of metal with neighbouring nitrogen nucleus.24 Such hyperfine splitting is absent in the EPR spectrum of ICp6–Cu which suggests that copper is not coordinated to pyrrole nitrogen. This is in agreement with a previous report on platinum(III) complex of hematoporphyrin where the EPR spectra of the complex having platinum attached to tetrapyrrole ring and the one in which platinum is attached to side chain carboxylic groups show similar difference.25 Further, the value of g-tensor and hyperfine splitting can be used to infer the coordination of copper with oxygen, nitrogen or sulphur based on the Peisach–Blumberg correlation.26 The value of g∥ (2.27) and A∥ (174 × 10−4 cm−1) obtained from the EPR spectrum are within the range of copper coordinating with oxygen atom. These findings unequivocally confirm that copper is attached to carboxylic groups. The nature of metal ligand bond can be predicted by calculating covalency parameter α2 from the following equation.
| αCu2 = −(A∥/0.036) + (g∥ − 2.003) + 3/7(g∥ − 2.003) + 0.04 | (1) |
A value of α2 = 0.5 indicates complete covalent nature and if the value is 1.0 it indicates ionic bonding.27 The value of α2 for ICp6–Cu is 0.82 which indicates that the nature of bonding between ligand and copper has some covalent character. Moreover, the value of α2 is higher than that reported for copper chlorophyll derivatives for Cu–N bond (0.63–0.68). This also suggests that the nature of copper–ligand bond in ICp6–Cu is significantly different.24
In Fig. 4, we show the FTIR spectra of ICp6–Cu and Cp6. The FTIR spectrum of Cp6 is very similar to the FTIR spectrum of Chlorin e6 reported by Gladkova et al.28 This is to be expected because the chemical structure of Ce6 is similar to Cp6 except the presence of one extra methylene (–CH2–) group at C15 position. The FTIR spectra of Cp6 in the region from 1300 cm−1 to 1750 cm−1 shows absorption bands due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and COO stretching vibrations of the carboxylic groups (Fig. 4b). The major changes observed upon complex formation are as follows (1) appearance of three new bands for ICp6–Cu, one at 1637 cm−1 which is characteristic of CO–metal stretch29,30 and two prominent bands at 1112 cm−1 and 1203 cm−1 due to CO stretching. In a previous report, the appearance of later two bands have been shown as direct proof for the formation of chelate of copper and zinc with C13 keto group of chlorophyll.30 (2) The COO vibration bands in Cp6 arise as a prominent band resolved into a peak at 1558 cm−1 and shoulders at 1578 cm−1 and 1596 cm−1. This band in ICp6–Cu is broad and one of the band at 1578 cm−1 disappeared. (3) The CO vibrations of C13 and C15 carboxylic group (at 1419 cm−1 and 1321 cm−1) are shifted to lower frequency at 1396 cm−1 and 1313 cm−1, respectively. These observations suggest that the two adjacent carboxylic groups at C13 and C15 position participate in formation of the metal coordination complex. The third carboxylic group at C17 propionic side chain is likely to remain attached to sodium because the region ∼1740 cm−1 which is characteristic of C
O and COO stretching vibrations of the carboxylic groups (Fig. 4b). The major changes observed upon complex formation are as follows (1) appearance of three new bands for ICp6–Cu, one at 1637 cm−1 which is characteristic of CO–metal stretch29,30 and two prominent bands at 1112 cm−1 and 1203 cm−1 due to CO stretching. In a previous report, the appearance of later two bands have been shown as direct proof for the formation of chelate of copper and zinc with C13 keto group of chlorophyll.30 (2) The COO vibration bands in Cp6 arise as a prominent band resolved into a peak at 1558 cm−1 and shoulders at 1578 cm−1 and 1596 cm−1. This band in ICp6–Cu is broad and one of the band at 1578 cm−1 disappeared. (3) The CO vibrations of C13 and C15 carboxylic group (at 1419 cm−1 and 1321 cm−1) are shifted to lower frequency at 1396 cm−1 and 1313 cm−1, respectively. These observations suggest that the two adjacent carboxylic groups at C13 and C15 position participate in formation of the metal coordination complex. The third carboxylic group at C17 propionic side chain is likely to remain attached to sodium because the region ∼1740 cm−1 which is characteristic of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrational band of this carboxylate show no change. In addition to above noted changes, the FTIR spectrum of ICp6–Cu also revealed an intense broad absorption band at ∼3400 cm−1 (Fig. 4c) which is expected to appear due to the presence of water molecules in the co-ordination sphere of copper.29
O vibrational band of this carboxylate show no change. In addition to above noted changes, the FTIR spectrum of ICp6–Cu also revealed an intense broad absorption band at ∼3400 cm−1 (Fig. 4c) which is expected to appear due to the presence of water molecules in the co-ordination sphere of copper.29
 | ||
| Fig. 4 FTIR spectra of Cp6 (black) and ICp6–Cu (red) plotted in the range of (a) 600–1250 cm−1, (b) 1300–1750 cm−1 and (c) 2800–4000 cm−1. | ||
The mass spectrum of ICp6–Cu is shown in Fig. 5. The molecular ion containing copper is identified by the characteristic isotopic splitting at 724.12 m/z and 726.12 m/z which represent loss of C17 carboxylate from the molecule. The largest peak observed at 701 m/z shows no such spliting and represents molecular ion with loss of metal and vinyl side group. This also rules out the possibility of iodination at vinyl group of Cp6. Previous reports on iodination of chlorophyll derivatives have shown that of the three meso positions at C5, C10, and C20, the meso position at C20 is relatively more reactive towards halogenation.31 The selective iodination of chlorophyll-a derivatives at C20 position has been reported in several studies.31–33 The replacement of meso-H with halogen in tetrapyrrole ring is identified by disappearance of characteristic proton peak in the 1H NMR spectrum. However, because the presence of paramagnetic copper leads to broadening of lines, a well resolved 1H NMR spectrum of ICp6–Cu could not be obtained. Based on evidence gathered from XRF (Fig. 2), EPR (Fig. 3), FTIR (Fig. 4), and HRMS (Fig. 5), the proposed chemical structure of ICp6–Cu is shown in Fig. 6. The site of iodination is indicated as most probable based on literature discussed above and needs to be confirmed.
1O2 and ROS generation efficiency of ICp6–Cu
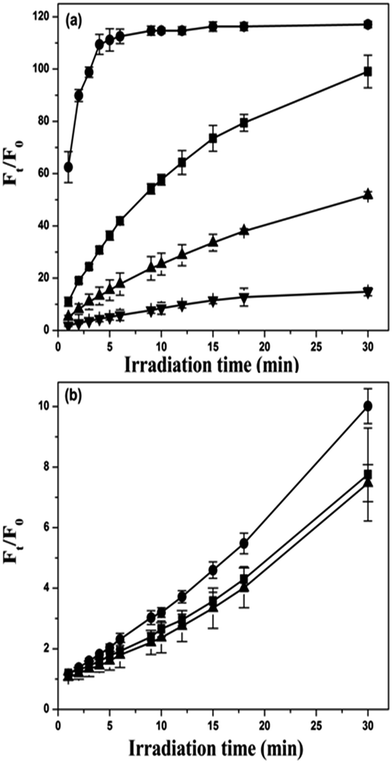
The 1O2 and ROS generation efficiency of ICp6–Cu was measured using three different fluorescence probes Singlet Oxygen Sensor green (SOSG), 2′,7′-dichlorodihydrofluorescin (DCFH) and 3′-(p-aminophenyl) fluroscein (APF). SOSG is specific for 1O2,34 DCFH detects total ROS35 and APF can detect ˙OH as well as 1O2.36 The 1O2 or ROS generated in photochemical reaction converts these probes into highly fluorescent species. The change in fluorescence intensity (Ft/F0) of SOSG and DCF as a function of irradiation time were plotted to compare the 1O2 and ROS generation capability of Cp6 (Fig. 7a) and ICp6–Cu (Fig. 7b). For ICp6–Cu, the relative increase in SOSG fluorescence is ∼10 times less than that for Cp6 (Fig. 7a and b), which suggest that as compared to Cp6, the efficiency of ICp6–Cu to produce 1O2 is reduced. It may be noted here that while the presence of heavy atom in the PS can improve the yield of 1O2 by increasing the efficiency of ISC from 1PS* to the 3PS,5,37 any shortening of excited state life time would result in a loss of 1O2 generation ability of the PS.38 Copper being paramagnetic results in reduction in excited state lifetime because of which most of the copper complex of porphyrin and chlorophyll derivatives reported in literature are photodynamically inactive.22,39 In contrast to copper, the presence of iodine on the PS usually increase the yield of 3PS and results in enhancement in the 1O2 yield.40,41 However, iodine depending on its position on the PS molecule can also results in shortening of 3PS life time and decrease in 1O2 yield.37 This is due to the fact that the orbital overlap between tetrapyrrole ring and iodine atom facilities non-radiative decay of the 3PS.37 Thus the decrease in 1O2 yield of ICp6–Cu can be attributed to the presence of both copper and iodine. It is also pertinent to note that PS with short life time of 3PS can be photodynamically active via type I process. For example, copper octaethylbenzochlorin despite short 3PS life time induce efficient photosensitization via type I process and has been found useful for PDT of cancer both in in vitro and in vivo studies.42The total ROS generation ability of ICp6–Cu was measured using fluorescence probe dichlorofluorescein (DCF). Fig. 7b show changes in DCF fluorescence induced by photoactivation of Cp6 or ICp6–Cu as a function of irradiation time. Here, the increase in DCF fluorescence is higher in Cp6 than for ICp6–Cu. However, since the yield of 1O2 for ICp6–Cu is low, the increase in DCF fluorescence is mainly due to generation of other ROS. Whereas for Cp6, the increase in DCF fluorescence is contributed predominantly by generation of 1O2 as seen in SOSG assay (Fig. 7b). These results show that the generation of other ROS by ICp6–Cu is substantially higher than the generation of 1O2. To confirm this, increase in DCF fluorescence was monitored in the presence of Sodium azide (NaN3), a quencher of 1O2. However, it was observed that the addition of NaN3 to DCF solution led to alteration in fluorescence of DCF which interfered with the measurement. In a previous report, fluorescence probe APF together with NaN3 and D2O (enhances life time of 1O2) has been effectively used to distinguish type I or type II photochemical mechanism of PDT drug.36 For Cp6 and ICp6–Cu, the changes in fluorescence of APF (Ft/F0) as a function of irradiation time is shown in Fig. 8a and b. For Cp6, the increase in fluorescence of APF is inhibited to ∼50% and ∼90% in the presence of 5 mM and 10 mM NaN3, respectively and it is enhanced by a factor of ∼4 in D2O (Fig. 8a). These results show that 1O2 is predominant species in photodynamic action of Cp6 which is also consistent with our previous report.43 In contrast, for ICp6–Cu induced increase in APF fluorescence is not significantly influenced by either NaN3 or D2O (Fig. 8b). These results clearly show that the photodynamic action of ICp6–Cu is mediated predominantly via type I process.
Phototoxicity of ICp6–Cu in cancer cells
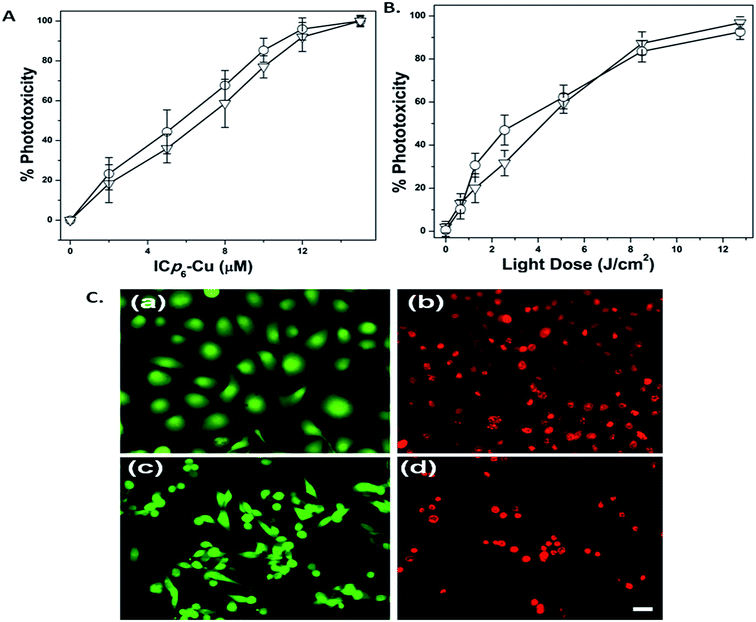
The dependence of phototoxicity of ICp6–Cu on the concentration and the light dose in two oral cancer cell lines (4451 and NT8e) is shown in Fig. 9A and B, respectively. Treatment of cells with various concentration of ICp6–Cu followed by light exposure at fixed light dose of 7 J cm−2 led to concentration dependent increase in phototoxicity (Fig. 9A). At 10 μM ICp6–Cu, the phototoxicity was ∼80% which increased further upto ∼95% at higher concentrations. However, there was also some (∼10%) dark toxicity in both cell lines beyond 10 μM ICp6–Cu (see ESI Table S1†). Based on this, 10 μM concentration of ICp6–Cu was selected to study the dependence of phototoxicity on light dose. As shown in Fig. 9B, PDT with 10 μM ICp6–Cu led to a dose dependent increase in phototoxicity. The phototoxicity was ∼60% at light dose of 5 J cm−2 and increased to ∼90% at 12 J cm−2. The changes in cell viability observed after staining with LIVE/DEAD kit is shown in Fig. 9C. In both NT8e and 4451, while all cells in control appear green (Fig. 9C(a) and(c)), the cells after PDT take up the red fluorescence due to loss of cell viability (Fig. 9C(b) and (d)). The morphological changes in two cell lines after PDT are shown in Fig. 10A. The cells in control appear healthy with intact cell membrane (Fig. 10A(a) and (c)). At 3 h after PDT (LD90 dose), almost all NT8e cells show formation of membrane blebs whereas, 4451 cells display damaged plasma membrane and swelling, indicated by arrows (Fig. 10A(b) and (d)). The percentages of live, apoptotic and necrotic cells in control and PDT treated cells for the two cell lines is shown in Fig. 10B. As expected, the mode of cell death in NT8e and 4451 cells occurred mainly via apoptosis and necrosis, respectively. This is due to the fact that the tumor suppressor p53 gene responsible for apoptotic death is wild type in NT8e cells whereas it is mutated in 4451 cells.44 These results are consistent with our earlier report on the mode of cell death induced by Cp6 and its histamine conjugate in these cell lines.44 The influence of quenchers of 1O2 (NaN3, L-histidine) and free radicals (dimethyl sulfoxide (DMSO), D-mannitol) on ICp6–Cu induced phototoxicity is shown in Fig. 11. As compared to control, the level of phototoxicity is significantly reduced in the presence of all the quenchers. Treatment of cells with quenchers alone under similar conditions did not result in any toxicity. It is important to mention that NaN3 and histidine can also quench ˙OH radicals.45 However, since the rate constant of NaN3 and histidine for reaction with ˙OH radicals is almost same, both should lead to inhibition of phototoxicity to the same extent. In contrast, the inhibition of phototoxicity by NaN3 is higher than that for histidine (Fig. 11) which correlates with their efficiency to quench 1O2.45 This suggests that in addition to free radicals, 1O2 also contributes to the phototoxicity of ICp6–Cu. The contribution of 1O2 to the phototoxicity of ICp6–Cu was not expected because studies on ROS generation in aqueous solution showed that the 1O2 generation ability of ICp6–Cu is very low (Fig. 7b and 8b). Here it is pertinent to note that the efficacy of the PS to generate 1O2 also depends on its environment. In aqueous solution, the PSs with higher hydrophobicity tend to aggregate and show decrease in 1O2 generation efficiency whereas the localization of PS in cellular membrane results in dis-aggregation and increase in photosensitization via type II process.46,47 A comparison of the absorption spectra of ICp6–Cu in aqueous medium and methanol (see ESI Fig. S1†) suggested that it is slightly aggregated in aqueous condition as indicated by significant blue shift and broadening of soret and Q bands (see ESI Table S2†). Further, the octanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water partition coefficient (Log P) of ICp6–Cu is 0.94 which is ∼2 times higher than Cp6 (see ESI Table S3†). Therefore due to higher hydrophobicity, ICp6–Cu can localize efficiently in cellular membrane.48 This and the fact that the life time of 1O2 in cellular environment is considerably longer (10 μs) as compared to that in aqueous media (3.5 μs)49 can explain the contribution of 1O2 to the phototoxicity for ICp6–Cu.
water partition coefficient (Log P) of ICp6–Cu is 0.94 which is ∼2 times higher than Cp6 (see ESI Table S3†). Therefore due to higher hydrophobicity, ICp6–Cu can localize efficiently in cellular membrane.48 This and the fact that the life time of 1O2 in cellular environment is considerably longer (10 μs) as compared to that in aqueous media (3.5 μs)49 can explain the contribution of 1O2 to the phototoxicity for ICp6–Cu.
Phototoxicity under hypoxic conditions
Availability of molecular oxygen is an important factor for the PDT efficacy of majority of PSs. It has been reported that low oxygen concentration can reduce the 1O2 yield and compromise the PDT efficacy.50–52 Alternatively, studies on PDT of human tumor xenografts with meta-tetrahydroxyphenylchlorin have shown that photochemical process leading to radical formation can work better under hypoxic condition.52 Several recent studies also showed that PS which act via type I photochemical mechanism induce better PDT efficacy under hypoxia. For example, phototoxicity mediated by methylene blue encapsulated in polymer nanoparticle53 and 5,10,15,20-tetrakis(meso-hydroxyphenyl)porphyrin incorporated in electron-rich poly(2-(diisopropylamino)ethyl methacrylate) micelles is not affected under hypoxia.54 Similarly, photodynamic release of doxorubicin induced by chondroitin sulfate conjugated Pheophorbide-a via type I mechanism led to higher toxicity under hypoxic conditions than that under normoxic conditions.55The effect of hypoxia on the phototoxicity of ICp6–Cu and Cp6 at LD50 and LD80 dose (for normoxic condition) is shown in Fig. 12. For Cp6, hypoxia led to decrease in phototoxicity by almost 1/2 (∼30% and 40%). In contrast, phototoxicity induced by ICp6–Cu under normoxic and hypoxic condition show no significant difference. Thus, as compared to Cp6, ICp6–Cu led to higher phototoxicity under hypoxic condition. It is important to mention that hypoxia has also been shown to affect the phototoxicity of bacteriopheophorbide which acts only via oxygen radicals, whereas the phototoxicity of Type II PS Npe6 was found to be unaffected.56 The possible reason for PDT efficacy of Npe6 under hypoxia was referred to superior ability of its 3PS to interact with oxygen.56 Although in our studies, as compared to Cp6 which also acts mainly via 1O2, the ability of ICp6–Cu to act via type I process appear important for its PDT efficacy under hypoxic condition.
Conclusions
To summarize, we have synthesized iodinated Chlorin p6 copper complex for PDT applications. EPR and FTIR studies suggest that copper is attached to the two adjacent carboxylic groups through co-ordination and the site of iodination may be meso position in the tetrapyrrole ring. Results of studies on the photodynamic efficacy of ICp6–Cu show that it is able to induce high phototoxicity in cancer cells and the phototoxicity is not affected significantly under hypoxic condition. In conclusion, results suggest that the novel copper complex is useful for PDT of cancer.Experimental section
Preparation of Cp6 and ICp6–Cu
Chlorophyll-a was extracted from dried spirulina powder and converted into Cp6 following the procedure described by Hoober et al.57 Cp6 was converted into ICp6–Cu according to our patent specifications (no. 4912/MUM/2015). In brief, the solution of Cp6 and CuI in methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (1
acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v) was mixed at equimolar concentration at room temperature. The solvent was partially evaporated to result in precipitation of the metal complex. The precipitates were collected by centrifugation (6000g, 10 min). The metal complex was further purified by silica column chromatography using methanol
10 v/v) was mixed at equimolar concentration at room temperature. The solvent was partially evaporated to result in precipitation of the metal complex. The precipitates were collected by centrifugation (6000g, 10 min). The metal complex was further purified by silica column chromatography using methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile solvent system. The stock solution of ICp6–Cu was prepared in ethanol
acetonitrile solvent system. The stock solution of ICp6–Cu was prepared in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG (400)
PEG (400)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (20
water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to prevent aggregation and contamination of the concentrated solution during storage.
50) to prevent aggregation and contamination of the concentrated solution during storage.
Spectroscopy
The UV/VIS absorption spectra of the Cp6 and ICp6–Cu were recorded from 350–750 nm, at 1 nm bandpass on a Cintra-20 spectrophotometer (GBC, Australia). The fluorescence emission spectra of Cp6 and ICp6–Cu were recorded on a Fluorolog-2 spectroflurometer (Spex, USA) by setting excitation wavelength at ∼400 nm, scan range from 500–700 nm and band pass of ∼1.7 nm and ∼3.7 nm for excitation and emission slits, respectively. FTIR spectra were recorded on a spectrometer model (FTLA 2000 MB 104) using powdered sample deposited on Zinc Selenide window. The X band EPR spectrum of ICp6–Cu was recorded on Spectrometer model JES – (Jeol, Japan) at 100 K. Methanol was used as solvent.The X-ray fluorescence of ICp6–Cu was monitored using microprobe XRF beamline (BL-16) of the synchrotron radiation source (Indus-2).58 For preparation of sample for XRF, 10 μl concentrated solution of ICp6–Cu in methanol was applied on a piece of Silicon wafer and air dried. Monochromatic X-ray of energy 12.0 keV (beam size 4 mm × 4 mm) was used to excite the fluorescence from the sample. XRF spectra were recorded using a Vortex energy-dispersive detector (SII NanoTechnology, USA).
Cell culture
Human oral cancer cell lines NT8e derived from upper aerodigestive tract (pyriform fossa) and 4451 derived from recurrent tumor in the lower jaw were obtained from ACTREC, Mumbai and INMAS, Delhi respectively. Both the cell lines were maintained in Dulbecco's Modified Eagle's Medium (DMEM) containing 4.5 g L−1 glucose, 4 mM L-glutamine, 25 mM of HEPES, antibiotics (streptomycin, nystatin, penicillin) and 10% fetal bovine serum. The cells were grown at 37 °C in a humidified incubator (Galaxy R, RS Biotech) under 5% CO2 + 95% air atmosphere. The cells were harvested by trypsinization and seeded in 35 mm Petri dishes or 96 well plate. After 18 h of incubation, the cells in exponential phase were used for the experiments.Irradiation
For assay of ROS generation and phototoxicity with ICp6–Cu, the irradiation was done using an LED lamp (Dia 4 cm) emitting red light (630 ± 20 nm). To ensure homogenous illumination, a diffuser glass plate was placed below the LED lamp. For similar studies with Cp6, a diode laser source (Thorlabs, USA) emitting red light (660 ± 5 nm) was used. Power of light was measured using a power meter model AN/2 (Ophir) and the power density was kept nearly same (∼3.5 mW cm−2) for both the light source.Generation of 1O2 and ROS
To determine the 1O2 and other ROS yield for ICp6–Cu and Cp6 we used three different fluorescence probes; SOSG and APF both from Invitrogen (USA) and 2′,7′-dichlorodihydrofluorescin-diacetate (DCFH-DA) from Sigma, USA. SOSG reacts specifically with 1O2 to result in formation of highly fluorescent endoperoxide product.34 DCFH-DA is a fluorescence probe for detection of intracellular generation of broad range of ROS such as O2˙− and ˙OH, hydrogen peroxide (H2O2) and 1O2. In cells, DCFH-DA is first converted enzymatically into DCFH which upon reaction with ROS is oxidized into green fluorescent DCF. For detection of ROS in solution, DCFH-DA is chemically converted into DCFH by hydrolysis according to the protocol described by Bourré et al.35 For this, DCFH-DA was mixed with 0.01 N NaOH in absolute ethanol and the solution was kept at room temperature for 30 min to allow hydrolysis. After neutralization with 25 mM sodium phosphate buffer (pH 7.4), the solution was stored on ice in dark until use. The fluorescence probe APF has been used to detect ˙OH and 1O2 both.36 APF upon reaction with ˙OH and 1O2 is converted to a fluorescent form due to the cleavage of the aminophenyl ring from fluorescein ring system.For monitoring generation of 1O2 and other ROS, ICp6–Cu or Cp6 (5 μM) was added to 3.5 ml sodium phosphate buffer (pH 7.4) in a plastic petridish (3.5 cm dia). After addition of the fluorescence probe, the solution was irradiated with narrow bandwidth light of appropriate wavelengths 630 ± 20 nm or 660 ± 5 nm for photoactivation of ICp6–Cu or Cp6 respectively, using light source described in section ‘Irradiation’. The irradiation was done in diffused dim light and the solution was continuously stirred during irradiation. At different time interval, 200 μl aliquot was withdrawn and fluorescence was measured on a spectrofluorometer. The wavelength used for fluorescence excitation of SOSG, DCF and APF were 485 nm, 488 nm and 490 nm respectively. The change in fluorescence intensity at 530 nm, 525 nm and 515 nm for SOSG, DCF and APF respectively were plotted as Ft/F0 vs. irradiation time, where F0 and Ft are the fluorescence intensity before and after irradiation.
Photosensitizer treatment
The growth medium from 35 mm petri dishes or 96 well plate containing cell monolayer was removed and replaced with fresh media containing ICp6–Cu. After incubation for 3 h at 37 °C in a humidified CO2 incubator in dark, the growth medium was aspirated and the cell monolayer was washed twice using plain medium (DMEM without serum). After adding fresh growth medium, the cells were subjected to photodynamic treatment. For concentration dependent PDT experiments, concentration of ICp6–Cu was varied from 2–15 μM and cells were irradiated with a fixed light dose of 7 J cm−2. For experiments where the light dose was varied, cells were treated with fixed concentration of 10 μM and irradiated with different light doses (0–12.7 J cm−2). For PDT under hypoxia, a fresh growth medium which was de-oxygenated by bubbling nitrogen gas for 1 h was used. The de-oxygenated medium was added to the cells after PS treatment and the cells were transferred to humidified incubator (ESCO) under controlled environment (1% O2 + 5% CO2) at 37 °C for 1 h. To maintain the hypoxia during irradiation, the 96 well plate containing cells was placed in a sealed glass chamber and the chamber was flushed with nitrogen. For studies with quenchers of ROS (NaN3, histidine, DMSO and mannitol), the cells prior to the photodynamic treatment were incubated for 1 h in growth medium containing specified concentration of each quencher and then irradiated at LD50 light dose.MTT assay
The dark and light induced cytotoxicity was determined using MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay.59 Briefly, at 24 h after PDT, the growth medium of the treated cells was replaced with plain medium containing 0.5 mg ml−1 MTT and the cells were incubated at 37 °C for 3 h in dark. After incubation, the medium was removed and 100 μl of DMSO was added and kept for 15 min to solubilise the formazan crystals formed within the cells. A microplate reader (Power Wave 340, Bio-tek instruments Inc., USA) was used to read the absorbance of the samples at 570 nm with reference wavelength at 690 nm. The percent phototoxicity was calculated with respect to the absorbance value in control samples. The cells treated with ICp6–Cu but not exposed to light were used as dark control. In each experiment at least four replicates were used and mean value was used to determine the percent cytotoxicity.Cell morphology and viability
PDT-induced cell damage and loss of cell viability was examined by bright field and fluorescence microscopy at 20× magnification using inverted microscope (Olympus, Japan). For cell viability, the cells after 24 h of PDT were stained with LIVE/DEAD cytotoxicity kit (Invitrogen, Molecular Probes, Eugene OR). The kit contains a green fluorescent dye calcein, which is retained in the cytoplasm by live cells and a DNA binding red fluorescent dye ethidium homodimer, that enter the cells only upon loss of plasma membrane integrity. After staining the cells were washed with phosphate buffered saline (PBS) and observed under fluorescence microscope using blue (450–480 nm) and green excitation (510–550 nm).Assessment of apoptosis and necrosis
To determine the percentage of cells undergoing apoptosis and necrosis, the cells after PDT were stained with Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 (10 μg ml−1) and propidium iodide (PI, 2.5 μg ml−1) for 5 min, washed twice with PBS and observed under inverted microscope (Olympus, Japan). PI is a cell impairment dye and can only stain the cells in which plasma membrane integrity is lost due to necrotic damage. Hoechst 33
342 (10 μg ml−1) and propidium iodide (PI, 2.5 μg ml−1) for 5 min, washed twice with PBS and observed under inverted microscope (Olympus, Japan). PI is a cell impairment dye and can only stain the cells in which plasma membrane integrity is lost due to necrotic damage. Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 is cell permeable, which stains nuclei and allows visualization of chromatin condensation and fragmentation of apoptotic cells. The blue fluorescence of Hoechst 33342 and red fluorescence of PI was visualized using excitation with 380–400 nm, barrier filter 440 nm. Images were recorded using a color CCD camera model ‘Prog Res Cfscan’ and a ProgRes Capture Pro Software (Jenoptik, Germany). A minimum of 500 cells were counted in each group and percentage of apoptotic, necrotic and live cells was calculated from the total number of cells counted.
342 is cell permeable, which stains nuclei and allows visualization of chromatin condensation and fragmentation of apoptotic cells. The blue fluorescence of Hoechst 33342 and red fluorescence of PI was visualized using excitation with 380–400 nm, barrier filter 440 nm. Images were recorded using a color CCD camera model ‘Prog Res Cfscan’ and a ProgRes Capture Pro Software (Jenoptik, Germany). A minimum of 500 cells were counted in each group and percentage of apoptotic, necrotic and live cells was calculated from the total number of cells counted.
Disclosure
Alok Dube, Paromita Sarbadhikary and Pradeep Kumar Gupta are named patent inventors for Indian Patent Application no. 4912/MUM/2015 titled ‘A metal complex of chlorophyll derivative for magnetic resonance imaging and photodynamic therapy’, filed on December 29, 2015.Acknowledgements
We are thankful to Dr G. S. Lodha and Dr M. K. Tiwari for helping with XRF measurement, Dr B. Jain for helping with FTIR measurements at our centre and Dr M. Shanmugam, IIT Bombay, Mumbai for EPR measurement. We also thank Dr B. S. Dwarakanath, INMAS, Delhi and Dr U. M. Warawdekar, ACTREC, Mumbai for providing oral cancer cell lines. S Paromita thanks Homi Bhabha National Institute, Mumbai for senior research fellowship.References
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Ca-Cancer J. Clin., 2011, 61, 250–281 CrossRef PubMed.
- R. R. Allison and K. Moghissi, Photodiagn. Photodyn. Ther., 2013, 10, 331–341 CrossRef CAS PubMed.
- A. P. Castano, T. N. Demidova and M. R. Hamblin, Photodiagn. Photodyn. Ther., 2004, 1, 279–293 CrossRef CAS PubMed.
- Y. M. Riyad, S. Naumov, S. Schastak, J. Griebel, A. Kahnt, T. Häupl, J. Neuhaus, B. Abel and R. Hermann, J. Phys. Chem. B, 2014, 118, 11646–11658 CrossRef CAS PubMed.
- L. B. Josefsen and R. W. Boyle, Met.-Based Drugs, 2008, 2008, 276109 Search PubMed.
- L. Benov, Med. Princ. Pract., 2015, 24, 14–28 CrossRef PubMed.
- R. K. Pandey, N. S. James, Y. Chen, J. Missert and M. Sajjad, Methods Mol. Biol., 2010, 635, 223–259 CAS.
- H. U. Rashid, M. N. Umar, K. Khan, M. N. Anjum and M. Yaseen, J. Struct. Chem., 2014, 55, 910–915 CrossRef.
- J. Gastaldo, Z. Bencokova, C. Massart, A. Joubert, J. Balosso, A. M. Charveta and N. Foraya, J. Synchrotron Radiat., 2011, 18, 456–463 CrossRef CAS PubMed.
- T. Ishizumi, K. Aizawa, T. Tsuchidaa, T. Okunakaa and H. Katoa, Photodiagn. Photodyn. Ther., 2004, 1, 295–301 CrossRef CAS PubMed.
- M. Miura, G. M. Morris, J. W. Hopewell, P. L. Micca, M. S. Makar, M. M. Nawrocky and M. W. Renner, Br. J. Radiol., 2012, 85, 443–450 CrossRef CAS PubMed.
- P. A. Waghorn, J. Labelled Compd. Radiopharm., 2014, 57, 304–309 CrossRef CAS PubMed.
- A. Dube, S. Sharma and P. K. Gupta, Oral Oncol., 2006, 42, 77–82 CrossRef CAS PubMed.
- A. Dube, S. Sharma and P. K. Gupta, Oral Oncol., 2011, 47, 467–471 CrossRef CAS PubMed.
- C. Santini, M. Pellei, V. Gandin, M. Porchia, F. Tisato and C. Marzano, Chem. Rev., 2014, 114, 815–862 CrossRef CAS PubMed.
- G. Hao, A. N. Singh, O. K. Oz and X. Sun, Curr. Radiopharm., 2011, 4, 109–121 CrossRef CAS PubMed.
- R. W. Miller, W. DeGraff, T. J. Kinsella and J. B. Mitchell, Int. J. Radiat. Oncol., Biol., Phys., 1987, 13, 1193–1197 CrossRef CAS.
- J. M. Widmark, Proc. Bayl. Univ. Med. Cent., 2007, 20, 408–417 Search PubMed.
- C. J. Anderson and R. Ferdani, Cancer Biother. Radiopharm., 2009, 24, 379–393 CrossRef CAS PubMed.
- B. Gutfilen and G. Valentini, BioMed Res. Int., 2014, 2014, 426892 Search PubMed.
- L. Prashanth, K. K. Kattapagari, R. T. Chitturi, V. R. Baddam and L. K. Prasad, J. NTR Univ. Health Sci., 2015, 4, 75–85 CrossRef.
- L. M. Moreira, A. Lima, R. R. S. Soares, V. R. Batistela, A. P. Gerola, N. Hioka, J. A. Bonacin, D. Severino, M. S. Baptista, A. E. H. Machado, M. R. Rodrigues, L. Codognotoa and H. P. M. Oliveira, J. Braz. Chem. Soc., 2009, 20, 1653–1658 CrossRef CAS.
- E. B. Fleischer, E. I. Choi, P. Hambright and A. Stose, Inorg. Chem., 1964, 3, 1284–1287 CrossRef CAS.
- Y. Nonomura, N. Yoshioka and H. Inoue, Inorg. Chim. Acta, 1994, 224, 181–184 CrossRef CAS.
- G. Momekov, M. Karaivanova, I. Ugrinova, E. Pasheva, G. Gencheva, D. Tsekova, S. Arpadjan and P. R. Bontchev, Invest. New Drugs, 2011, 29, 742–751 CrossRef CAS PubMed.
- S. K. Hoffmann, J. Goslar, S. Lijewski and A. Zalewska, J. Magn. Reson., 2013, 236, 7–14 CrossRef CAS PubMed.
- N. Raman, S. Ravichandran and C. Thangaraja, J. Chem. Sci., 2004, 116, 215–219 CrossRef CAS.
- O. L. Gladkova, M. V. Parkhats, A. N. Gorbachova and S. N. Terekhov, Spectrochim. Acta, Part A, 2010, 76, 388–394 CrossRef CAS PubMed.
- M. Q. Zhang, Y. C. Zhu, J. G. Wu, P. Shi, R. W. Deng and Z. N. Chen, Chem. Pap., 2001, 55, 202–205 CAS.
- J. Petrovic, G. Nikolic and D. Markovic, J. Serb. Chem. Soc., 2006, 71, 501–512 CrossRef CAS.
- M. Ethirajan, P. Joshi, W. H. William, K. Ohkubo, K. S. Fukuzumi and R. K. Pandey, Org. Lett., 2011, 13, 1956–1959 CrossRef CAS PubMed.
- H. Tamiaki, N. Arik, S. Yasuda, T. Miyatak and T. Oba, Tetrahedron, 2014, 70, 9768–9775 CrossRef CAS.
- H. Tamiaki, Y. Kotegawa and K. Mizutani, Bioorg. Med. Chem. Lett., 2008, 18, 6037–6040 CrossRef CAS PubMed.
- A. Gollmer, J. Arnberg, F. H. Blaikie, B. W. Pedersen, T. Breitenbach, K. Daasbjerg, M. Glasius and P. R. Ogilby, Photochem. Photobiol., 2011, 87, 671–679 CrossRef CAS PubMed.
- L. Bourré, S. Thibaut, A. Briffaud, N. Rousset, S. Eléouet, Y. Lajat and T. Patrice, J. Photochem. Photobiol., B, 2002, 67, 23–31 CrossRef.
- M. Price, J. J. Reiners, A. M. Santiago and D. Kessel, Photochem. Photobiol., 2009, 85, 1177–1181 CrossRef CAS PubMed.
- F. Nifiatis, J. C. Athas, K. D. D. Gunaratne, Y. Gurung, K. M. Monette and P. J. Shivokevich, Open Spectrosc. J., 2011, 5, 1–12 CrossRef CAS.
- S. Mathai, T. A. Smith and K. P. Ghiggino, Photochem. Photobiol. Sci., 2007, 6, 995–1002 CAS.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233, 351–371 CrossRef.
- A. C. Serra, M. Pineiro, A. M. Rocha Gonsalves, M. Abrantes, M. Laranjo, A. C. Santos and M. F. Botelho, J. Photochem. Photobiol., B, 2008, 92, 59–65 CrossRef CAS PubMed.
- S. H. Lim, C. Thivierge, P. Nowak-Sliwinska, J. Han, H. van den Bergh, G. Wagnières, K. Burgess and H. B. Lee, J. Med. Chem., 2010, 53, 2865–2874 CrossRef CAS PubMed.
- G. M. Garbo, V. H. Fingar, T. J. Wieman, E. B. Noakes 3rd, P. S. Haydon, P. B. Cerrito, D. H. Kessel and A. R. Morgan, Photochem. Photobiol., 1998, 68, 561–568 CrossRef CAS PubMed.
- B. Bose and A. Dube, J. Photochem. Photobiol., B, 2008, 93, 32–35 CrossRef CAS PubMed.
- A. Parihar, A. Dube and P. K. Gupta, Cancer Chemother. Pharmacol., 2011, 68, 359–369 CrossRef CAS PubMed.
- S. Basu-Modak and R. M. Tyrrell, Cancer Res., 1993, 53, 4505–4510 CAS.
- L. I. Grossweiner, A. S. Patel and J. B. Grossweiner, Photochem. Photobiol., 1982, 36, 159–167 CrossRef CAS PubMed.
- H. Mojzisova, S. Bonneau, P. Maillard, K. Berg and D. Brault, Photochem. Photobiol. Sci., 2009, 8, 778–787 CAS.
- F. M. Engelmann, S. V. Rocha, H. E. Toma, K. Araki and M. S. Baptista, Int. J. Pharm., 2007, 329, 12–18 CrossRef CAS PubMed.
- J. Baier, M. Maier, R. Engl, M. Landthaler and W. Bäumler, J. Phys. Chem. B, 2005, 109, 3041–3046 CrossRef CAS PubMed.
- J. Moan and S. Sommer, Cancer Res., 1985, 45, 1608–1610 CAS.
- J. D. Chapman, C. C. Stobbe, M. R. Arnfield, R. Santus, J. Lee and M. S. McPhee, Radiat. Res., 1991, 126, 73–79 CrossRef CAS PubMed.
- V. O. Melnikova, L. N. Bezdetnaya, D. Brault, A. Y. Potapenko and F. Guillemin, Int. J. Cancer, 2000, 88, 798–803 CrossRef CAS PubMed.
- M. Usacheva, S. K. Swaminathan, A. R. Kirtane and J. Panyam, Mol. Pharm., 2014, 11, 3186–3195 CrossRef CAS PubMed.
- H. Ding, H. Yu, Y. Dong, R. Tian, G. Huang, D. A. Boothman, B. D. Sumer and J. Gao, J. Controlled Release, 2011, 156, 276–280 CrossRef CAS PubMed.
- W. Park, B. C. Bae and K. Na, Biomaterials, 2016, 77, 227–234 CrossRef CAS PubMed.
- M. Price, L. Heilbrun and D. Kessel, Photochem. Photobiol., 2013, 89, 683–686 CrossRef CAS PubMed.
- J. K. Hoober, T. W. Sery and N. Yamamoto, Photochem. Photobiol., 1988, 48, 579–582 CrossRef CAS PubMed.
- M. K. Tiwari, P. Gupta, A. K. Sinha, S. R. Kane, A. K. Singh, S. R. Garg, C. K. Garg, G. S. Lodha and S. K. Deb, J. Synchrotron Radiat., 2013, 20, 386–389 CrossRef CAS PubMed.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: percent cytotoxicity of ICp6–Cu in dark at 10–15 μM concentration; Fig. S1: absorption spectra of ICp6–Cu in buffer and methanol; Table S2: Absorption characteristics of ICp6–Cu in methanol and buffer; Table S3: octanol/water partition coefficient of Cp6 and ICp6–Cu. See DOI: 10.1039/c6ra14026b |
| This journal is © The Royal Society of Chemistry 2016 |