Antiviral mechanism study of gossypol and its Schiff base derivatives based on reactive oxygen species (ROS)†
Abstract
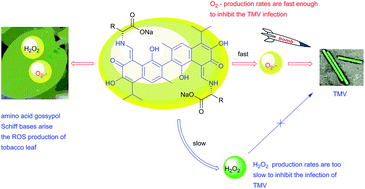
A series of amino acid gossypol Schiff bases were designed, synthesized and evaluated for their anti-TMV activities, and a new method of preparing hydrophilic amino acid gossypol Schiff bases, which are difficult to prepare using existing methods, was developed. Most of these compounds displayed good activities. Structure–activity relationship studies revealed that both the carboxy group and R group in amino acids are important to their anti-TMV activities. Further mechanism studies based on ROS-producing ability indicated that the O2˙− production rates of gossypol Schiff bases showed a positive correlation with their anti-TMV activity, suggesting that O2˙− is more important than H2O2 at the primary stage of TMV inoculation. However, the gossypol Schiff bases derived from D and L-amino acids, respectively, showed different anti-TMV activities (4 > 16, 6 > 17, 7 > 18, 11 > 19) although they had the same ROS-producing abilities. Both the D and L-amino acid gossypol Schiff bases stimulate O2˙− and H2O2 production in tobacco leaves. The current study provides fundamental support for the development of gossypol alkaloids as potential inhibitors of plant viruses.

 Please wait while we load your content...
Please wait while we load your content...