DFT studies of Ni cluster on graphene surface: effect of CO2 activation†
He Xuab,
Wei Chu ab,
Wenjing Sunc,
Chengfa Jiang*ab and
Zhongqing Liu*a
ab,
Wenjing Sunc,
Chengfa Jiang*ab and
Zhongqing Liu*a
aSchool of Chemical Engineering Sichuan University, Chengdu 610065, China. E-mail: liuzq_hgxy@scu.edu.cn; jiangcf@scu.edu.cn; Fax: +86-28-8540-0422
bSichuan Provincial Environmental Protection Center for Catalytic Materials Engineering Technology, Chengdu 610064, China
cChina-America Cancer Research Institute, Key Laboratory for Medical Molecular Diagnostics of Guangdong Province, Guangdong Medical University, Dongguan, Guangdong 523808, China
First published on 4th October 2016
Abstract
CO2 capture, storage, sequestration (CCS), and utilization (CCU) are regarded as the most effective way to slow the pace of global warming. In our present work, the structural stability and catalytic properties for adsorbing CO2 on the surface of isolated Ni clusters and 5–8–5 monovacancy graphene (MGr) supported Ni clusters have been investigated using density functional theory. The results show that, with the increasing of atomic number, the structure of isolated Ni clusters tended to be stable and the structural parameters approached to that of metal crystal. In addition, when Ni clusters deposited on the MGr surface, the Ni–Ni bond generally elongated, indicating activation of the isolated metal clusters by graphene. Adsorption energies of CO2 onto Ni4 cluster and MGr were −1.80 and −1.35 eV, respectively, while the value reached −2.72 eV when CO2 adsorbed on Ni4 cluster modified with MGr (Nix/MGr). The phenomenon indicates that the adsorption capacity of CO2 were significantly improved by depositing Ni clusters onto MGr surface and the system tended to be more stable. According to the analyses of Mulliken charge, electrostatic potential (EP) and partial density of states (PDOS), more electrons transferred from Nix cluster to the region of CO2 and MGr when CO2 adsorbed on Nix/MGr. The mechanism was proposed for CO2 adsorption on Nix/MGr at the molecular level. It is of great significance to devise high activity catalyst of CO2 storage and hydrocarbon production from CO2.
I Introduction
Due to large number of burning fossil fuels such as oil, coal, etc., or deforestation, large amounts of carbon dioxide have been produced, causing a global greenhouse effect. Carbon capture, storage, sequestration and utilization are regarded as the most effective measure to reduce CO2 in atmosphere, or at least to control greenhouse effect.1–5 Among them, adsorption technologies using metal oxide, zeolite, or carbon materials like carbon nanotubes (CNTs) and graphene as a candidate of adsorbent beds have been experimentally and theoretically investigated.6Additionally, other studies involve using carbon dioxide in a certain reaction,7–10 for example, hydrocarbon production from CO2. Hydrocarbons can be used as fuel components and this approach will help relieve energy crisis. The dominating mechanism for this process is the Sabatier reaction:11 CO2 + 4H = CH4 + 2H2O (−252 kJ mol−1). The first step of this reaction is to adsorb and activate CO2. However, as a linear molecule, CO2 has no polarity and is hard to activate due to its high thermodynamic stability. If the linear structure of CO2 transfers into a bent structure, the activation energy barrier will be significantly reduced.12 It is a generally accepted fact that CO2 activation on transition metal surfaces is structure sensitive, that is, different surface structures have different activity capacities.13–15 The catalytic activities of CO2 on the surface of Co, Ni,8 Cu,16 Pt,17 Pd,18 Fe,19 CeO2,12 Ni4/γ-Al2O3 (ref. 20) and Pd/DOH zeolite21 have been explored in recent years, and the results show that the CO2 activation energy can be greatly decreased by lengthening the C–O bond, and increasing the bending degree of molecules, owing to a large number of electron transfer from CO2 molecular to the metal atom.22,23 It has been verified that the reaction of CO2 on Ni also involves the formation of the bent intermediate CO2δ−, which can greatly reduce the reaction energy barrier.24 So metal Ni is the most widely used in the study of CO2 activation mechanism.
Recently, metal-decorated carbon materials have attracted more attention as they exhibit excellent stability and durability under harsh conditions.25–27 Johll28 explored Fe, Co, and Ni adatoms and dimers adsorbed on graphene and the results showed adatoms bonding weakly to graphene with binding energies ranging from 0.2 to 1.4 eV. At the same time, the computational insight into the activity of transition metal clusters has been hotly investigated. Deshpande9 carried out a stable seven-atom pentagonal bipyramidal Cu cluster acting as a biomimetic CO2 hydration catalyst and the result exhibited the metal clusters as a good catalyst for CO2 capture. Pacchioni29 investigated electronic interactions and charge transfer of metal atoms and clusters on oxide surfaces. Additionally, Sun8 carried out experimental and theoretical investigations on the interaction between palladium nanoparticles/clusters and functionalized carbon nanotubes for Heck synthesis. The results reveal the defects on carbon material promoted the redistribution of electrons, enhanced the metal–C interaction, and further decreased the size of metal nanoparticle. Hahn30 considered the effects of CO2 adsorption on CeO2 (111) surface by depositing Ni cluster.
However, up to now the investigation of CO2 adsorption on MGr surface deposited with Ni clusters for carbon utilization application has been few. In our work, monolayer monovacancy grapheme was selected mainly because that defect on graphene could reallocate electrons between the metal and the C atom of CO2, thereby promoting interaction of metal and graphene. And grapheme is comprised of benzene rings, a characteristic beneficial for electron transfer on the surface. First, the activities and stabilities of isolated Nix (x = 1, 2, 3, 4, 5, 6, 7, 9, 10) cluster in the gas phase were taken into account and identified. Then, the adsorption behavior of Ni clusters on MGr surface and CO2 on the surfaces of Ni4 cluster, MGr, and Ni4/MGr were simulated and analyzed in terms of energetic (binding energy), structural (Ni–Ni bond length and gyration radius), and electronic properties (Mulliken charge, EP and PDOS). Finally, the adsorption mechanism of CO2 activated on Ni4/MGr was proposed and a systematic comparison of CO2 activation capacity on isolated Ni clusters, pristine MGr, and Ni4/MGr were listed, which aimed to illuminate the influence of CO2 adsorption capacity over supported metal Ni clusters on the surface of monolayer graphene at the molecule level based on the density functional theory (DFT). Consequently, the work provides fundamental insight for CO2 utilization by depositing Ni clusters onto MGr, and potentially provides the synthesis of new carbon-based materials.
II Theoretical methods
All the calculations were carried out in DMol3 package of Materials Studio31,32 based on density functional theory (DFT). Generalized gradient approximation (GGA)33 with Perdew–Burke–Ernzerhof (PBE) functional was used to model the electron exchange and correlation interaction. All electron double numerical atomic orbitals augmented by p- and d-polarization functions (DNP)34,35 were set as the basis set in the simulation of isolate Ni clusters, and orbital cutoff quality was set at fine. The thermal smearing level was set at 0.005 to improve the convergence, and basis set superposition error (BSSE) was not taken into account, as the numerical basis sets implemented in DMol3 minimized or even eliminated basis set superposition error.36 Spin-polarization was considered in all calculations, the ground state of Ni cluster structures was obtained from the minimum in the total energy and preferred spin multiplicity, and the atomic positions of all models were allowed to relax in all calculations.Simulation of isolate Ni clusters in the gas phase consisting of one to ten atoms was investigated first. The energy of formation Eform of that Ni clusters was calculated according to eqn (1):
| Eform = (Ecluster − nNiENi,g)/nNi | (1) |
The binding energy EB of Ni clusters adsorbed on MGr has been calculated in analogy to the formation energy according to eqn (2):
| EB = [Eslab+Nix − (nNiENi,g + Eslab)]/nNi | (2) |
Simulation of MGr was implemented in a periodically repeated box of 12.3 × 12.3 × 20 Å. One layer of 5 × 5 × 1 periodic super unit cell was employed to avoid interaction between periodic unit cells. Monovacancy graphene is defined as MGr in this paper, which is a vacancy structure of one C-atom missing in the perfect grapheme and leaving each of three neighboring C-atoms with sp2 dangling bonds. Plane-wave electronic density functional theory with long range dispersion interaction corrections DFT-D37–39 was considered to balance computational efficiency and accuracy, and the lattice parameter of graphene was optimized to be 4.12 Å, which was in well agreement with a previous report40 and theoretical calculations.41 All calculations consisting of graphene was performed with periodic boundary conditions, and a vacuum region of 20 Å between periodic basal planes was introduced along the [111] direction for avoiding interactions with adjacent plane.
The adsorption behavior of CO2 onto the surface of respective substrate was simulated, and the adsorption energy of CO2 (Eads) was calculated according to eqn (3):
| Eads = Eslab*+CO2 − Eslab* − ECO2 | (3) |
III Results discussion
A. Isolated Nix clusters
Isolated Nix clusters were optimized (Fig. 1) firstly from two atoms to ten atoms in gas phase. The three-dimensional (3D) structure of Ni cluster is more stable than the flat structure,42 thus 3D models as small metal clusters were chosen for the following research. Total energy (Etotal), dNi–Ni, atomization energies (EA) and formation energies (Eform) were calculated, data shown in Table 1.| Molecule | m | Etotal/(a.u.) | dNi–Ni/nm | EA/(eV) | Eform/(eV) |
|---|---|---|---|---|---|
| a m is the number of Ni atom in the cluster modes.b EA = [E(Nix) − xE(Ni)], Eform = EA/x. | |||||
| CO2 | — | −188.48 | — | — | — |
| Ni1 | — | −1508.08 | — | — | — |
| Ni2 | 1 | −3016.26 | 2.12 | −2.70 | −1.35 |
| Ni3 | 3 | −4524.42 | 2.24 | −5.08 | −1.69 |
| Ni4 | 4 | −6032.60 | 2.31 | −7.87 | −1.97 |
| Ni5 | 5 | −7540.80 | 2.34 | −10.58 | −2.12 |
| Ni6 | 6 | −9049.00 | 2.35 | −13.80 | −2.30 |
| Ni7 | 7 | −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 557.21 557.21 |
2.38 | −17.23 | −2.46 |
| Ni9 | 9 | −13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 573.64 573.64 |
2.37 | −24.52 | −2.72 |
| Ni10 | 10 | −15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081.82 081.82 |
2.38 | −27.66 | −2.77 |
The results indicate that total energies Etotal and atomization energies EA decreased with the increasing number of Ni atoms. The formation energies Eform ranged from −1.35 eV to −2.72 eV with Ni clusters getting larger, which agreed with the previous result43,44 that metal clusters became more stable with increasing Ni atoms, while their structural parameters approaching that of metal crystals. The dNi–Ni increased and Eform decreased with increasing Ni atom numbers, as described in Fig. 2. For the Ni3 cluster, the equilateral triangle with bond length of 2.24 Å was the most stable configuration, with atomization energy EA = −5.08 eV and formation energy Eform = −1.69 eV. Our results are in agreement with that of Park's experimental results that the Ni3 cluster preferred to choose triangle as the ground state structure.45 These results are also consistent with ref. 42 and 46–48 where the atomization energy ranged from −3.4 eV to −5.4 eV, but slightly different from those references in an allowable deviation that generating different functions and parameters. For Ni5, a nearly perfect trigonal bipyramid was found, and the Eform = 2.12 eV agreed with the previous report of 2.40 eV.49 Additionally, the approximate value of formation energy for Ni9 and Ni10 showed the tendency of formation energy slowing down and stabilizing gradually, indicating the structure parameters of Ni clusters turning to that of metal crystal.
 | ||
| Fig. 2 Average Ni–Ni bond length dNi–Ni (solid symbols) and formation energy Eform (empty symbols) of isolated Ni clusters (gas phase). | ||
A similar trend for the formation energy was observed for the average Ni–Ni bond length (dNi–Ni). It increased from 2.12 Å in the Ni dimer to 2.38 Å for Ni10, which was consistent with the previously calculation studies by using DFT30,42,48 where the Ni–Ni bond elongated from 2.06 to 2.10 Å in the Ni dimer to more than 2.40 Å in a Ni cluster with 15 atoms. The amplification of dNi–Ni curve became smooth and steady up to seven Ni atoms, and the variation tendency of bond length was in good agreement with experimental measurement of 2.15 Å.50
B. Nix cluster adsorbed on MGr: geometry and stability
Geometry and stable configurations of Nix clusters (x = 1–7, 9, 10) on MGr are showed in Fig. 3. It is found that the Ni clusters are out of shape and Ni–C bond formed in the interface of Ni cluster and MGr. For the system of one Ni atom deposited on MGr surface, the elevation (h) out of the graphene sheet is 1.50 Å, and the binding energy is 0.73 eV, whose structure is given in ESI (Fig. S1†) and those results are well in agreement with the previous report.51The structure of Ni cluster with no more than ten atoms deposited on MGr surface was optimized, and every Ni atom in the triplet state for all configurations, the curve of binding energy is shown in Fig. 4. A lower binding energy corresponds to a more stable system. The trend of stability for Ni clusters deposited on MGr is similar to isolated Ni clusters, in which the binding energy decreases with the number of Ni atoms. EB increases quickly when the value of n is no more than 4 and then it becomes stable gradually, which indicates the system of Nix/MGr tended to be stable. The binding energies EB changed from −0.73 eV to −3.59 eV, referring to chemisorption.
 | ||
| Fig. 4 Binding energy EB (eV) of Ni cluster adsorb on MGr as a function of the number of Ni atoms in the cluster for 3D configuration. | ||
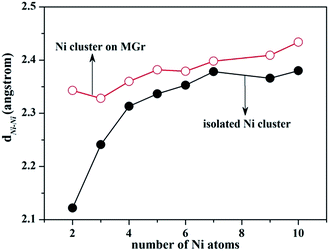 | ||
| Fig. 5 Average Ni–Ni bond length dNi–Ni of isolated Ni cluster (solid circle symbols) and Ni cluster adsorbed on MGr (empty circle symbols). | ||
A frequently used parameter to classify shapes is the radius of gyration (Rg), and the morphology of a particular Ni cluster at a given time can be defined as the equation:30
![[r with combining macron]](https://www.rsc.org/images/entities/i_char_0072_0304.gif) was the mean position of the Ni cluster, N was the number of Ni atoms.
was the mean position of the Ni cluster, N was the number of Ni atoms.
Radius of gyration refers to the distribution of the components of an object around an axis. In terms of mass moment of inertia, it is the perpendicular distance from the axis of rotation to a point mass that gives an equivalent inertia to the original object(s). The gyration radius were calculated and tabulated in Table 2, using linear regression (QSAR) procedures based on the linear combination of atomic orbital and molecular orbital method. A smaller radius of gyration indicates a more compact structure of the cluster. The linear relationship of their normalized radius of gyration 
 30 and binding energy (EB) both as pristine Nix and adsorbed on MGr is displayed in Fig. 6.
30 and binding energy (EB) both as pristine Nix and adsorbed on MGr is displayed in Fig. 6.
| Configuration | EF (ev) | EB (ev) | dNi–Ni (Å) | Rg (Å) | ||
|---|---|---|---|---|---|---|
| Gas phase | On MGr | Gas phase | On MGr | |||
| 1Ni | — | −0.73 | — | — | — | — |
| 2Ni | −1.35 | −1.52 | 2.12 | 2.34 | 1.06 | 1.17 |
| 3Ni | −1.69 | −1.73 | 2.24 | 2.33 | 1.29 | 1.34 |
| 4Ni | −1.97 | −2.68 | 2.31 | 2.36 | 1.42 | 1.46 |
| 5Ni | −2.12 | −2.99 | 2.34 | 2.38 | 1.60 | 1.62 |
| 6Ni | −2.30 | −3.07 | 2.35 | 2.38 | 1.66 | 1.68 |
| 7Ni | −2.46 | −3.12 | 2.38 | 2.40 | 1.84 | 1.88 |
| 9Ni | −2.72 | −3.24 | 2.37 | 2.41 | 2.01 | 2.08 |
| 10Ni | −2.72 | −3.59 | 2.38 | 2.43 | 2.13 | 2.19 |
The result indicates that the binding energy displays a linear trend with increasing  . According to the equation:
. According to the equation:  , the value of intercept at the hypothetical limit of
, the value of intercept at the hypothetical limit of  indicates the maximum binding energy of Ni clusters deposited on the surface of MGr, EB,0 = −4.63 eV, the slope k = 5.43 eV Å−1, and Pearson's r2 = 93.3%. Additionally, the thermodynamically unfavorable condition EB ≥ 0 eV, in which Ni cluster cannot be adsorbed, can be estimated by the maximal normalized radius of gyration
indicates the maximum binding energy of Ni clusters deposited on the surface of MGr, EB,0 = −4.63 eV, the slope k = 5.43 eV Å−1, and Pearson's r2 = 93.3%. Additionally, the thermodynamically unfavorable condition EB ≥ 0 eV, in which Ni cluster cannot be adsorbed, can be estimated by the maximal normalized radius of gyration  .
.
C. CO2 adsorption on Ni/MGr systems
| Substrate | EB,CO2 (eV) | dC–O(1) (Å) | dC–O(2) (Å) | αO–C–O (°) |
|---|---|---|---|---|
| Ni4 cluster | −1.80 eV | 1.263 | 1.262 | 136.26 |
| MGr | −1.35 eV | 1.376 | 1.377 | 107.86 |
| Ni4/MGr | −2.72 eV | 1.250 | 1.243 | 140.31 |
CO2 adsorption on Ni4/MGr surface is showed in Fig. 8(c and f). Compared with Ni4 and MGr, the strongest interaction was that of Ni4/MGr with a binding energy of −2.72 eV (Tables 2 and S1†), where the one C–O bond was elongated to 1.250 Å, the other was elongated to 1.243 Å, and the O–C–O angle was found to be 140.31°. Compared with isolated Ni cluster and original MGr, CO2 on Nix/MGr had the highest absorption energy, indicating the supported Ni clusters could significantly improve the adsorption energy of CO2 by changing its structure.
D. Electronic structure
Mulliken charge, electrostatic potential and PDOS (Fig. 9 and S5†) of Ni4 and CO2 were calculated when CO2 adsorbed in the stable configurations on the surface of models discussed above. Electron transfer between adsorbate and adsorbent materials by Mulliken charge analysis is listed in Table 4, and electrostatic potential generated by Mulliken charge analysis is showed in Fig. 8(g–i), the isosurfaces were constructed at 0.0675 e−1 Å−3. Negative value lobes of electrostatic potential indicate the region of gain electrons, whereas positive value lobes represent lost electrons. As shown in the Fig. 8, the negative charge enriched in the region of O atoms, positive charge enriched in the region of Ni atoms, indicating the C, O and neighboring Ni atoms activated obviously. This leads to forming a higher electron density surrounding CO2 compared to that of CO2 in the gas phase, which further indicates that accumulated electrons in these regions can easily help form a new bond of C–Ni or two Ni–O bonds. | ||
| Fig. 9 PDOS of CO2 and Ni4 on different systems, (a) pure CO2, (b–d) CO2 on the Ni4, MGr, and Ni4/MGr surface, and (e) isolated Ni cluster, (f–h) Ni4 on the CO2–Ni4, Ni4/MGr, and CO2–Ni4/MGr surface. | ||
| Atom | Ni4 | CO2–Ni4 | CO2–MGr | Ni4–MGr | CO2–Ni4/MGr |
|---|---|---|---|---|---|
| a Represents the sum of Mulliken atomic charges of CO2 on different models.b Represents the sum of Mulliken atomic charges of Ni4 on different models.c Represents the sum of Mulliken atomic charges of MGr on different models. | |||||
| Ni1 | −0.001 | 0.171 | — | 0.219 | 0.172 |
| Ni2 | 0.005 | 0.047 | — | 0.246 | 0.281 |
| Ni3 | −0.003 | 0.167 | — | 0.205 | 0.321 |
| Ni4 | −0.001 | 0.048 | — | 0.004 | 0.120 |
| O1 | — | −0.432 | −0.423 | — | −0.408 |
| C1 | — | 0.431 | 0.394 | — | 0.443 |
| O2 | — | −0.433 | −0.450 | — | −0.386 |
| Suma | 0 | −0.434 | −0.479 | — | −0.351 |
| Sumb | 0 | 0.434 | — | 0.674 | 0.885 |
| Sumc | 0 | — | 0.479 | −0.674 | −0.534 |
For Mulliken charge, positive value denotes the gain of electrons compared to that of the original structure, while negative value represents a loss. The sum of Mulliken atomic charges of CO2 on different models in Table 4 shows that the CO2 molecule gained electrons, Ni4 cluster losed electrons, and MGr surface gained electrons as CO2. Electron transfer from Ni cluster to CO2 and the surface of MGr, indicating Ni cluster acting as a Lewis base donating its electron to the MGr and CO2, while MGr and CO2 molecule acting as Lewis acids withdrawing electron density from the surface of Ni cluster. The adsorption of small molecules onto the surface of MGr changes the charge distribution. Compared with CO2 adsorbed on isolated Ni4 and MGr, Ni4 cluster in the system of CO2–Ni4/MGr has the maximum amount of donated electrons of 0.885, indicating the strongest activation level of Ni4. This phenomenon is consistent with the results manifested by binding energy and the electrostatic potential. The mutual transfer of multi-electron indicates a strong activation capacity and a significant change on its structure, which will greatly enhance the adsorption capacity of CO2.
The projected density of states (PDOS) of CO2 and Ni4 cluster on different systems are displayed in Fig. 9 and S5.† Compared with the PDOS of pure CO2 [Fig. 9(a)], the peaks of s and p orbitals for CO2 become wider and lower when CO2 adsorb on Ni cluster, MGr and Ni4/MGr systems, especially the p orbits near the Fermi level, which indicates that CO2 was activated and chemical adsorption occurred between CO2 and those adsorbents. The phenomenon of p orbitals in chart (b) and (c) moved towards to right indicates total energy in the two systems becoming lower compared with original structure, in agreement with the theory reaction occurred toward a lower entropy direction, namely, the system is more stable after adsorption of CO2.
Fig. 9(f–h) depicts the PDOS of Ni4 cluster in the system of CO2–Ni4, Ni4–MGr and CO2–Ni4/MGr, respectively. Compared with isolated Ni cluster [Fig. 9(e)], the occupied 3d and p orbitals have a significantly change in Fig. 9(f–h), with peaks generally becoming lower and wider, which reveals the electron cloud around the Ni cluster was out of shape and electrons were bound by MGr and CO2 when adsorption occurred. Besides, Fermi level moved towards to the direction of low energy in Fig. 9(g and h), indicating the system becomes stable owing to the adsorption occurred between Ni cluster, CO2 molecule and MGr. This adsorption lead to a change in the PDOS and it suggests that s and p orbitals of CO2 and Ni atoms turned relatively larger, but there was little influence on d orbital.
IV Conclusions
DFT calculations were carried out to investigate the adsorption microcosmic mechanism and fundamental properties of CO2 adsorption on supported Ni clusters on monovacancy graphene surface for catalytic applications. The results show that, Ni cluster tended to be a metal crystal structure with the increasing of atomic numbers, the average bond length of Ni–Ni tended to be a stable value with the size up to seven atoms, and the formation energy verged to a steady state with nine Ni atoms.Binding energy and structural properties of Ni clusters on MGr were investigated by using DFT. It was found that binding energy became stable with increasing Ni atom numbers, and stabilized with increasing cluster size up to ten atoms adsorbed on MGr. The average bond length of Ni–Ni in isolated state and in MGr-supported Ni clusters with the increasing number of Ni atoms elongated to 2.38 Å and 2.43 Å, respectively. The value of maximal normalized radius of gyration  indicates small Ni particles were stable on the MGr surface when radius of gyration exceeded 0.854 Å. The radius of gyration showed a linear correlation with binding energy of Ni cluster.
indicates small Ni particles were stable on the MGr surface when radius of gyration exceeded 0.854 Å. The radius of gyration showed a linear correlation with binding energy of Ni cluster.
CO2 adsorbed on different systems (isolated Nix cluster, MGr, and Nix/MGr) yielded a significantly lower binding energy (−2.72 eV) of CO2 on MGr supported Ni4 cluster than on isolated Ni4 cluster (−1.80 eV). The catalytic activities of CO2 were enhanced on the surface of MGr by supporting Ni4 cluster. Additionally, Mulliken charge, electrostatic potential (EP) and partial density of states (PDOS) were also analyzed based on a change of the electronic structure of the Ni cluster deposited on MGr surface. More electrons transferred from Ni4 cluster to the region of MGr supported metal system when CO2 was adsorbed. The mechanism was proposed on CO2 adsorption onto MGr deposited with Ni clusters at the molecular level. Our work affords important significance for CO2 storage, capture and catalytic reaction of hydrocarbon production from CO2.
Abbreviation
| MGr | Monovacancy graphene |
| Nix/MGr | Nickel cluster deposited on the surface of MGr |
| EP | Electrostatic potential |
| PDOS | Partial density of states |
Acknowledgements
This work was supported by National Basic Research Program of China (973 Program) (2011CB201202) & the National Natural Science Foundation of China (No. 21376154).Notes and references
- J.-J. Mo, Y. Xue, X.-Q. Liu, N.-X. Qiu, W. Chu and H.-P. Xie, Surf. Sci., 2013, 616, 85–92 CrossRef CAS.
- Y.-X. Zhao, H.-L. Ding and Q. Zhong, Appl. Surf. Sci., 2012, 258, 4301–4307 CrossRef CAS.
- Y.-Y. Liu and J. Wilcox, Int. J. Coal Geol., 2012, 104, 83–95 CrossRef CAS.
- Y.-Y. Liu and J. Wilcox, Environ. Sci. Technol., 2013, 47, 95–101 CrossRef CAS PubMed.
- X. Huang, W. Chu, W.-J. Sun, C.-F. Jiang, Y.-Y. Feng and Y. Xue, Appl. Surf. Sci., 2014, 299, 162–169 CrossRef CAS.
- K.-J. Lee and S.-J. Kim, Bull. Korean Chem. Soc., 2013, 34, 3022–3026 CrossRef CAS.
- A.-H. Liu, R. Ma, C. Song, Z. Z. Yang, A. Yu, Y. Cai, L.-N. He, Y.-N. Zhao, B. Yu and Q.-W. Song, Angew. Chem., Int. Ed. Engl., 2012, 51, 11306–11310 CrossRef CAS PubMed.
- W. J. Sun, Z.-Q. Liu, C.-F. Jiang, Y. Xue, W. Chu and X.-S. Zhao, Catal. Today, 2013, 212, 206–214 CrossRef CAS.
- M. Verma, K. B. S. Kumar and P. A. Deshpande, J. Phys. Chem. C, 2016, 120, 5577–5584 CAS.
- P. A. Deshpande, Chem. Eng. Sci., 2016, 145, 294–298 CrossRef CAS.
- S. Ohmi, C. Kobayashi, I. Kashiwagi, C. Ohshima, H. Ishiwara and H. Iwai, J. Electrochem. Soc., 2003, 150, F134 CrossRef CAS.
- K. R. Hahn, M. Iannuzzi, A. P. Seitsonen and J. Hutter, J. Phys. Chem. C, 2013, 117, 1701–1711 CAS.
- S.-G. Wang, D.-B. Cao, Y.-W. Li, J.-G. Wang and H.-J. Jiao, J. Phys. Chem. B, 2005, 109, 18956–18963 CrossRef CAS PubMed.
- M. C. J. Bradford and M. A. Vannice, Catal. Rev., 1999, 41, 1–42 CAS.
- F. Solymosi, J. Mol. Catal., 1991, 65, 337–358 CrossRef CAS.
- G.-C. Wang, L. Jiang, Y. Morikawa, J. Nakamura, Z.-S. Cai, Y.-M. Pan and X.-Z. Zhao, Surf. Sci., 2004, 570, 205–217 CrossRef CAS.
- P. R. Norton and P. J. Richards, Surf. Sci., 1975, 49, 567–576 CrossRef CAS.
- D. Ehrlich, S. Wohlrab, J. Wambach, H. Kuhlenbeck and H.-J. Freund, Vacuum, 1990, 41, 157–160 CrossRef CAS.
- M. H. Nassir, J. Vac. Sci. Technol., A, 1993, 11, 2104 CAS.
- Y.-X. Pan, C.-J. Liu and Q.-F. Ge, J. Catal., 2010, 272, 227–234 CrossRef CAS.
- D. Smykowski, B. Szyja and J. Szczygiel, J. Mol. Graphics Modell., 2013, 41, 89–96 CrossRef CAS PubMed.
- H. J. Freund and M. W. Roberts, Surf. Sci. Rep., 1996, 25, 225–273 CrossRef.
- S.-G. Wang, X.-Y. Liao, D.-B. Cao, C.-F. Huo, Y.-W. Li, J.-G. Wang and H.-J. Jiao, J. Phys. Chem. C, 2007, 111, 16934–16940 CAS.
- X.-L. Ding, V. Pagan, M. Peressi and F. Ancilotto, Mater. Sci. Eng., C, 2007, 27, 1355–1359 CrossRef CAS.
- L. Chen, K.-L. Yang, H.-T. Liu and X.-L. Wang, Carbon, 2008, 46, 2137–2139 CrossRef CAS.
- J. Y. Kim, Y. Jo, S.-K. Kook, S. Lee and H. C. Choi, J. Mol. Catal. A: Chem., 2010, 323, 28–32 CrossRef CAS.
- A. Villa, D. Wang, N. Dimitratos, D.-S. Su, V. Trevisan and L. Prati, Catal. Today, 2010, 150, 8–15 CrossRef CAS.
- H. Johll, H. C. Kang and E. S. Tok, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 245416 CrossRef.
- G. Pacchioni, Phys. Chem. Chem. Phys., 2013, 15, 1737–1757 RSC.
- K. R. Hahn, A. P. Seitsonen, M. Iannuzzi and J. Hutter, ChemCatChem, 2015, 7, 625–634 CrossRef CAS.
- B. Delley, J. Chem. Phys., 1990, 92, 508 CrossRef CAS.
- B. Delley, J. Chem. Phys., 2000, 113, 7756 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(16), 533–539 Search PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- Y. Inada and H. Orita, J. Comput. Chem., 2008, 29, 225–232 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2004, 25, 1463–1473 CrossRef CAS PubMed.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- L. Pauling, The Nature of the Chemical Bond, Cornell University Press, 1960, p. 18 Search PubMed.
- J. Thakur, M. K. Kashyap, H. S. Saini and A. H. Reshak, J. Alloys Compd., 2016, 663, 100–106 CrossRef CAS.
- Q. L. Lu, Q. Q. Luo, L. L. Chen and J. G. Wan, Eur. Phys. J. D, 2010, 61, 389–396 CrossRef.
- Y. Jiang, W. Chu, C.-F. Jiang and Y.-H. Wang, Acta Phys.-Chim. Sin., 2007, 23, 1723–1727 CAS.
- V. Bertin, E. Agacino, R. López-Rendon and E. Poulain, J. Mol. Struct.: THEOCHEM, 2006, 769, 243–248 CrossRef CAS.
- E. K. Parks, L. Zhu, J. Ho and S. J. Riley, J. Chem. Phys., 1994, 100, 7206–7222 CrossRef CAS.
- C. Ashman, S. N. Khanna and M. R. Pederson, Chem. Phys. Lett., 2003, 368, 257–261 CrossRef CAS.
- F. A. Reuse and S. N. Khanna, Chem. Phys. Lett., 1995, 234, 77–81 CrossRef CAS.
- B. V. Reddy, S. K. Nayak, S. N. Khanna, B. K. Rao and P. Jena, J. Phys. Chem. A, 1998, 102, 1748–1759 CrossRef CAS.
- G. Rollmann, S. Sahoo and P. Entel, Eur. Phys. J. D, 2011, 61, 389–396 CrossRef.
- J. C. Pinegar, J. D. Langenberg, C. A. Arrington, E. M. Spain and M. D. Morse, J. Chem. Phys., 1995, 102, 666–674 CrossRef CAS.
- F. Nasehnia and M. Seifi, Mod. Phys. Lett. B, 2014, 28, 1450237 CrossRef CAS.
- Y.-Y. Liu and J. Wilcox, Environ. Sci. Technol., 2011, 45, 809–814 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The optimized structures of perfect-graphene sheet and one Ni atom deposited on graphene were listed, PDOS of Ni atom and C atom were analyzed in different adsorption system, stable configurations of CO2 adsorbed on Nix cluster and CO2 adsorbed on MGr surface deposition of Nix cluster were calculated, sum of density of states projected on the atoms of the CO2 molecule adsorbed on isolated Ni4, on MGr surface and on the Ni4/MGr system. See DOI: 10.1039/c6ra14009b |
| This journal is © The Royal Society of Chemistry 2016 |







