Synthesis of silsesquioxane-based element-block amphiphiles and their self-assembly in water†
Abstract
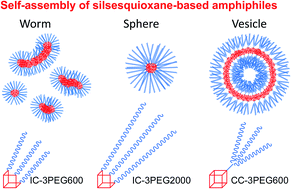
Incompletely condensed (IC) and completely condensed (CC) polyhedral oligomeric silsesquioxanes (POSSs) tethered with hydrophilic poly(ethylene glycol) (PEG) chains were synthesized and used as novel organic–inorganic amphiphilic element-block molecules toward self-assembly nanomaterials. The association behavior of these element-block molecules in water can be controlled based on their chemical structures. The eight PEG chains-containing CC-POSS, with a structure of the hydrophobic CC-POSS center covered with hydrophilic PEG chains, is hydrophilic and can molecularly dissolve in pure water. IC-POSS, which carries three PEG chains with a molecular weight of 2000, is an amphiphilic compound and forms spherical micelles consisting of a hydrophobic IC-POSS core and hydrophilic PEG chain shell. IC-POSS, which carries three PEG chains with a molecular weight of 600, forms polydisperse worm-shaped micelle aggregates, because the hydrophilic PEG chains are very short for stable dispersion of independent spherical micelles. Amphiphilic CC-POSS, which carries branched PEG chains with a molecular weight of 600, forms a vesicle structure, although IC-POSS carrying three PEG chains forms solid micelles in spite of the same PEG number and length. These results strongly indicate that the length of the PEG chain and the shape of the POSS head group play a crucial role in determining the self-assembly structures.

 Please wait while we load your content...
Please wait while we load your content...