Cu(ii)-catalyzed cross-dehydrogenative coupling reaction of N′-acyl arylhydrazines and phosphites†
Abstract
A novel Cu(II)-catalyzed cross-dehydrogenative coupling reaction of N′-aryl acylhydrazines and dialkyl phosphites has been developed for the synthesis of phosphorylhydrazides by using NMO as an external oxidant and AgNO3 as additive. Various N′-aryl acylhydrazines were mildly and regioselectively converted to corresponding phosphorylhydrazides with good to excellent yields.
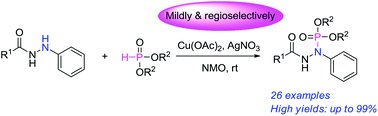

 Please wait while we load your content...
Please wait while we load your content...