Self-organized helical superstructure of photonic cellulose loaded with upconversion nanoparticles showing modulated luminescence†
Abstract
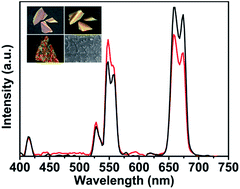
The ability to manipulate the color output of nanomaterials is important for applications like optoelectronic devices, light emitting display and lasers. Here, a self-organized helical superstructure of photonic cellulose loaded with upconversion nanoparticles of NaYF4:Yb,Er has been realized. The modulated upconversion luminescence of the photonic composite film of cellulose-NaYF4:Yb,Er has been demonstrated with the mechanism proposed.

 Please wait while we load your content...
Please wait while we load your content...