Metal ion binding of the third and fourth domains of Slc11a1 in a model membrane
Haiyan Qi*,
Wanxia Tang,
Liming Bai and
Lidi Gao
College of Chemistry and Chemical Engineering, Qiqihar University, No. 42, Wenhua Street, Qiqihar, P. R. China. E-mail: qhy120@sina.com
First published on 6th September 2016
Abstract
Differential scanning calorimetric (DSC) experiments have shown that the ability of third and fourth transmembrane domains of Slc11a1 to perturb DMPC model membranes is affected by metal ions. The results indicate the third and fourth transmembrane Domains of Slc11a1 can bind with metal ions. Our findings may be helpful in understanding the transport mechanism of Slc11a1.
Slc11a1 (Solute carrier 11a1, formerly Nramp1; where Nramp stands for natural-resistance-associated macrophage protein) consisting of 12 transmembrane domains belongs to the Slc11 (solute carrier 11) or Nramp (natural resistance-associated macrophage protein) family of proton-coupled metal ion transporters1–4 that function in diverse organisms from bacteria to humans and play a major role in metal ion homeostasis.5–9 As a pH-dependent divalent cation transporter, Slc11a1 has been proved to transport Fe2+, Mn2+, Co2+ and, to a lesser extent, Zn2+.10–12 Structure-function studies of Slc11a1 homologues using mutagenesis approaches have revealed that the segment TM4 and TM3 may be involved in metal ion binding and could contribute to the formation of the Slc11a cation translocation pathway. Both disease-causing mutations in Slc11a1 occurring at glycine 169 (G169D) and Slc11a2 occurring at glycine 185 (G185R) locate within TM4. In the TMs of Slc11 proteins, TM3 was found to be important for specific aspects of transport. The mutation from Glu to Ala at position 154 (E154A) within the putative TM3 of Slc11a2 caused complete loss of function.13,14 Similar results were also found in MntH, the conservative mutations of E102D and E102Q (Glu102 is analogous to Glu154 in Slc11a2 and Glu139 in Slc11a1) resulted in a complete loss of transport activity.15 Metal ions are essential cofactors for a wealth of biological processes, including oxidative phosphorylation, gene regulation and free-radical homeostasis. Although the significance of Slc11a1 in divalent metal ion transport is well established, the transport mechanism remains poorly understood.
In a previous study, DSC experiments aimed at that the incorporation of both peptides into lipids leads to a substantial decrease in the enthalpy and cooperativity of the main transition, suggesting that the peptides penetrate into the phospholipid bilayers and accordingly disturb the packing of the hydrophobic chains of the lipids via hydrophobic and van der Waal's interactions between peptides and membrane.16,17 However, direct proof of metal ion binding to the TM3 and TM4 has not been reported and the transport mechanism is still obscure.
In the present study the different role of Fe2+ (was prepared fresh in deionized water + L-(+)-ascorbic acid sodium salt to prevent oxidation of Fe2+), Ba2+, Mn2+, Cr3+ ions in affecting the interaction of TM3 and TM4 of Slc11a1 with DMPC model membranes was investigated by DSC experiments. It may verify the metal ion binding to the two segments of Slc11a1. Our findings may be helpful in understanding the transport mechanism of Slc11a1.
In particular DMPC–TM3–ion and DMPC–TM4–ion systems were investigated to evaluate how the endothermic melting enthalpy (ΔH) and the temperature (Tm) of the gel–liquid crystal phase transition of the membrane were affected by the peptide–metal complexes. In a control experiment a certain amount of TM3 and TM4 solved in HFIP were separately added into the chloroform/methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution of DMPC then the mixtures were dried under N2 gas.18 The films were vortexed with buffer (pH 5.5) at a peptide/lipid molar fraction of 0.0125. The lipid dispersion was then extruded to form large unilamellar vesicles (LUV) according to a procedure described elsewhere.19,20 The final concentration of TM3, TM4 were 18.75 μM. The systems were then stepwise titrated with an excess of Fe2+ performing a DSC scan (heating rate = 0.5 °C min−1) after each addition. The same experiments were carried out in parallel using Ba2+, Mn2+, Cr3+ instead of Fe2+.
1) solution of DMPC then the mixtures were dried under N2 gas.18 The films were vortexed with buffer (pH 5.5) at a peptide/lipid molar fraction of 0.0125. The lipid dispersion was then extruded to form large unilamellar vesicles (LUV) according to a procedure described elsewhere.19,20 The final concentration of TM3, TM4 were 18.75 μM. The systems were then stepwise titrated with an excess of Fe2+ performing a DSC scan (heating rate = 0.5 °C min−1) after each addition. The same experiments were carried out in parallel using Ba2+, Mn2+, Cr3+ instead of Fe2+.
Addition of metal ions modify the Tm and enthalpy of the lipid transition. The Tm and the ΔH of DMPC/TM4 mixtures were 22.04 ± 0.05 °C and 12.6 ± 2 kJ mol. The lipid transition was similar to the control, within the limits of experimental error. Then the system was stepwise titrated with an excess of Fe2+ performing, after each addition, a DSC run (Fig. 1). Addition of Fe2+ modified the Tm of the lipid transition and the enthalpy. A plot of the measured Tm and ΔH vs. the ions: TM4 molar ratio (Fig. 2), has shown that the Tm and ΔH were increased, when ions added. In addition, the extent of increase of the transition temperature and the endothermic melting enthalpy varies with joining dissimilar ions. When Fe2+ and Mn2+ were added, the Tm largely increased (transition temperature Tm has increased about 1.73 °C), but the percentages of the increase in ΔH (increased 9.28% and 10.2% respectively) were very small compared to other two ions (the percentages of the change in ΔH were 19.3% and 28.9%). It was revealed that influences results more than any ions. When Ba2+ was added, the change of Tm was the least (about 0.86 °C) and the percentages of the increase in ΔH was the greatest. Though DSC as a tool to investigate the peptide-induced perturbation of lipid bilayers, the enthalpy change observed during the lipid main transition is mainly ascribable to the packing efficiency of the hydrocarbon tails. The peptide-induced decrease of the transition enthalpy of the bilayer may thus be related to the extent of the interaction between guest molecules and the core of lipid membranes. Moreover, Tm is more sensitive to interactions involving the lipid head groups, and increases when the membrane surface is involved in the interaction with the guest peptide. Our results suggests that certain ions addition inhibits the interaction between TM4 and DMPC. Maybe the presence of the ions not only affect the effects of the presence of the peptide on the physical state of the membrane, but also the topological arrangement of the peptide inserted into a lipid matrix. And then explained the Fe2+ and Mn2+ metal ions could bind with TM4. On the other hand, the binding abilities of Ba2+, Cr3+ with TM4 were not pronounced. Whilst previously obtained results have evidenced that both the peptide is inserted in the phospholipid bilayer21,22 the present results show that ions addition may reduce perturbation of TM4 to the lipid packing.
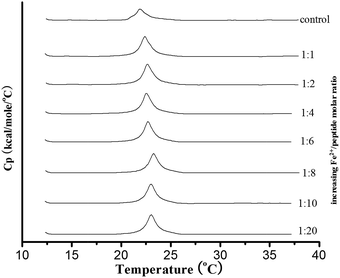 | ||
| Fig. 1 DSC curves of DMPC–TM4–Fe2+ mixtures obtained at different TM4/Fe2+ molar ratios. The first curve represents the DSC profile of the DMPC–TM4 system without metal, reported as a control. | ||
The same experiments were carried out in parallel using TM3 instead of TM4 and the results were reported in Fig. 3. The Tm and the ΔH of DMPC/TM3 mixtures were 22.70 ± 0.05 °C and 13.7 ± 2 kJ mol. Differently from DMPC/TM4, when the four metal ions were added, the Tm of TM3 was also increasing, but the degree of change of ΔTm were all smaller compared with TM4. In addition, the extents of increase of Tm (increased between 1.18–1.34 °C) were similar whether varies with joining dissimilar ions were joined, except Fe2+ (Tm has increased about 1.70 °C). Besides when Fe2+ was added, the percentages of the change in ΔH is the smallest (the percentages of the change in ΔH was 13.7%), the endothermic melting enthalpy increased significantly (the percentages of the change in ΔH were 29.9–35.2%) while the other ions were added. It showing that Fe2+ could binding with TM3, the other metal ions may binding to TM3. But the binding ability was bad compared with TM4.
Conclusions
The results here reported provide the first experimental evidence that the binding of TM3, TM4 of Slc11a1 with metal ions Fe2+, Ba2+, Mn2+, Cr3+ from the isolated peptides using a DSC method based on the temperature and the endothermic melting enthalpy ΔH influenced by metal ions. Two factors may play a role in this remarkable difference in affecting the properties of DMPC observed for ions: (i) the different affinity of the four metals for the peptide; (ii) the different roles of the segments TM4 and TM3 in metal ion binding and contribute to the formation of the Slc11a cation translocation pathway. The TM4 domain binded with Fe2+, Mn2+ and TM3 domain binded with Fe2+ more strongly than others. The binding ability of TM4 was also stronger than TM3. The conclusions is conformable to physiologic research. TM3, TM4 may be involved in metal ion binding and could contribute to the formation of the Slc11a1 cation translocation pathway. These are the first direct proof of metal ions binding to the TM3 and TM4.Acknowledgements
This work was financially supported by the Program for the Nature Science Foundation in Heilongjiang Province (B201314), (B2015016) and Young Teachers Scientific Research in Qiqihar University (2014k-Z12).Notes and references
- S. M. Vidal, D. Malo, K. Vogan, E. Skamene and P. Gros, Cell, 1993, 73, 469 CrossRef CAS PubMed.
- F. Supek, L. Supekova, H. Nelson and N. Nelson, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 5105 CrossRef CAS.
- H. Gunshin, B. Mackenzie, U. V. Berger, Y. Gunshin, M. F. Romero, W. F. Boron, S. Nussberger, J. L. Gollan and M. A. Hediger, Nature, 1997, 388, 482 CrossRef CAS PubMed.
- M. D. Fleming, C. C. Trenor 3rd, M. A. Su, D. Foernzler, D. R. Beier, W. F. Dietrich and N. C. Andrews, Nat. Genet., 1997, 16, 383 CAS.
- N. Nelson, EMBO J., 1999, 18, 4361 CrossRef CAS PubMed.
- J. R. Forbes and P. Gros, Trends Microbiol., 2001, 9, 397 CrossRef CAS PubMed.
- A. Van Ho, D. M. Ward and J. Kaplan, Annu. Rev. Microbiol., 2002, 56, 237 CrossRef CAS PubMed.
- T. Goswami, A. Rolfs and M. A. Hediger, Biochem. Cell Biol., 2002, 80, 679 CrossRef CAS PubMed.
- D. Agranoff, L. Collins, D. Kehres, T. Harrison, M. Maguire and S. Krishna, Biochem. J., 2005, 385, 225 CrossRef CAS PubMed.
- T. Goswami, A. Bhattacharjee, P. Babal, S. Searle, E. Moore, M. Li and J. M. Blackwell, Biochem. J., 2001, 354, 511 CrossRef CAS PubMed.
- P. G. Atkinson and C. H. Barton, Immunology, 1999, 96, 656 CrossRef CAS PubMed.
- J. R. Forbes and P. Gros, Blood, 2003, 102, 1884 CrossRef CAS PubMed.
- P. Courville, R. Chaloupka and M. F. Cellier, Biochem. Cell Biol., 2006, 84, 960 CrossRef CAS PubMed.
- S. Lam-Yuk-Tseung, G. Govoni, J. Forbes and P. Gros, Blood, 2003, 101, 3699 CrossRef CAS PubMed.
- H. A. Haemig and R. J. Brooker, J. Membr. Biol., 2004, 201, 97 CrossRef CAS PubMed.
- G. W. J. Seto, S. Marwaha, D. M. Kobewka, R. N. A. H. Lewis, F. Separovic and R. N. McElhaney, Biochim. Biophys. Acta, 2007, 1768, 2787 CrossRef CAS PubMed.
- P. Garidel, M. Rappolt, A. B. Schromm, J. Howe, K. Lohner, J. Andrä, M. H. J. Koche and K. Brandenburg, Biochim. Biophys. Acta, 2005, 1715, 122 CrossRef CAS PubMed.
- S. Majee and A. Chakrabarti, Biochem. Pharmacol., 1999, 57, 981 CrossRef CAS PubMed.
- D. Grasso, D. Milardi, C. La Rosa and E. Rizzarelli, New J. Chem., 2001, 25, 1543 RSC.
- D. Grasso, D. Milardi, V. Guantieri, C. La Rosa and E. Rizzarelli, New J. Chem., 2003, 27, 359 RSC.
- H. Y. Qi, L. Yang, R. Xue, Y. D. Song, S. Wang and F. Li, Biopolymers, 2009, 92, 52 CrossRef CAS PubMed.
- H. Y. Qi, Y. Wang, H. T. Chu, W. H. Wang and Q. D. Mao, Spectrochim. Acta, Part A, 2014, 122, 82 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |


