Specific electronic absorptions of alternate layered nanostructures of two metal oxides synthesized via a thiol–ene click reaction†
Abstract
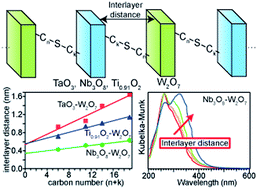
Alternate layered nanostructures are synthesized with thiol-modified niobate nanosheets or tantalate nanosheets and alkene-modified tungstate nanosheets via a thiol–ene click reaction. The stacking distance of the nanosheets is increased linearly with the increase of the carbon number contained in the bridging chain, and controlled within the nanometer order by changing the carbon number generated by the thiol–ene click reaction. Different behaviors observed in the absorption spectra of the two combinations are discussed in terms of the electronic interaction between the neighboring nanosheets. The absorption peak attributed to the bandgap transition of tungstate in the absorption spectrum of the alternate layered nanostructure of niobate and tungstate is blue-shifted with the decrease of the stacking distance. This observation leads us to conclude that the density of states of the tungstate nanosheets is changed by formation of a p–n junction in the alternate layered structure. On the other hand, when the stacking distance of the alternate layered nanostructure of tantalate and tungstate is varied, there are no shifts of the absorption peak attributed to the bandgap transition of tungstate. This result indicates that the electronic structure of the tantalate nanosheets and the tungstate nanosheets in the alternate layered structure are independent. It is discovered that the electronic structures of the alternate stacking structures constructed by thiol–ene click reaction can be modified by changing the stacking distance of the alternate layered nanostructures, controlled by changing the carbon number.

 Please wait while we load your content...
Please wait while we load your content...