Synthesis and characterization of mesoporous silica monoliths with polystyrene homopolymers as porogens
Chaoyue Wang,
Lei Li and
Sixun Zheng*
Department of Polymer Science and Engineering, The State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: szheng@sjtu.edu.cn; Fax: +86-21-54741297; Tel: +86-21-54743278
First published on 1st November 2016
Abstract
In this work, we explored the preparation of mesoporous silica materials by using polystyrene (PS) homopolymers as the porogens. First, a series of polystyrene (PS) homopolymers with various molecular weights were synthesized via atom transfer radical polymerization (ATRP). The single ends of these PS homopolymers were then functionalized with hydroxyl groups via copper(I)-catalyzed cycloaddition reaction, and these can then react with 3-isocyanatopropyltriethoxysilane to afford the triethoxysilane-terminated PS homopolymers. In the presence of the triethoxysilane-terminated PS homopolymers, the sol–gel process of tetraethoxysilane (TEOS) was carried out to afford a series of organic–inorganic silica gels. These silica gels were then used as precursors to obtain the mesoporous silica materials via the removal of PS microdomains with pyrolysis at elevated temperatures. Transmission electron microscopy (TEM) showed that all the silica materials displayed spherical or cylindrical nanopores with the size of the nanopores being 10–30 nm. The mesoporous structure was further evidenced by the measurement of specific surface areas with nitrogen sorption experiments. It was found that the specific surface areas can be adjusted in terms of the contents and molecular weights of the PS homopolymers. The generation of nanopores in the silica materials is ascribed to the formation of the PS microdomains in the organic–inorganic silica gels as revealed by small angle X-ray scattering (SAXS). It is proposed that the functionalization of the PS chain ends confined the reaction-induced phase separation of the PS homopolymers on the nanometer scale in the organic–inorganic silica gels. The approach reported in this work was in marked contrast to the utilization of amphiphiles as the porogens of the mesoporous silica.
Introduction
Mesoporous silica materials have potential applications in catalysis, sorption, separation, sensors and biotechnologies owing to their high surface area and well-defined nanoporous structures.1–6 In the past years, there has been persistent interest to explore new and efficient approaches to prepare mesoporous silica materials. Generally, porous silica materials can be prepared with sol–gel processing of organic alkoxysilanes such as tetraethoxysilane (TEOS) or tetramethoxysilane (TMOS) in the presence of a variety of porogens.7–11 The sol–gel process results in the microdomains of the porogens being trapped in the silica gels; the subsequent removal of the labile microdomains leaves the pores in the resulting silica materials. Depending on size and distribution of porogen microdomains, mesoporous porous silica can be prepared with ordered or disordered structures as well as tunable pore sizes. In ample literature, amphiphilic molecules such as low molecular surfactants and block copolymers have been employed as the soft templates to direct the formation of the nanostructures in silica gels and further to access the corresponding mesoporous structures in silica.11–18 It has been recognized that by the use of amphiphilic molecules the formation of nanostructures in silica gels would follow self-assembly or evaporation-induced self-assembly mechanism.11,19–22 The size of pores and specific surface area of mesoporous silica materials are closely related to the morphologies of the microdomains trapped in the silica gels, which can be modulated with compositions, molecular weights and architectures of amphiphilic molecules.13,23 By using low molecular surfactants, the pores in the silica materials are generally controlled to have the size as small as a couple of nanometers. Recently, it has been reported that sizes of pores can be further adjusted by external adding so-called pore-expanding agents such as aromatic hydrocarbons,24,25 long chain alkanes26–28 and/or auxiliary alkyl surfactants.29 These compounds are microdomain-soluble and thus can well swell the microdomains in the silica gels. The utilization of amphiphilic block copolymers allows the preparation of the mesoporous silica materials with the pore sizes as large as tens of nanometers. Nonetheless, it is critical to use appropriate block copolymers for the control over the mesoporosity of silica materials. To suppress macroscopic phase separation in the sol–gel process, amphiphilic block copolymers are required to have both silica-philic and silica-phobic subchains. Generally, the affinity of copolymer subchains with silica is achieved through physical interactions (e.g., hydrogen bonding). For instance, poly(ethylene oxide) (PEO) is frequently used as the subchains of a great number of amphiphilic block copolymers suitable for the preparation of mesoporous silica materials. It has been realized that PEO chains can form the intermolecular hydrogen bonding interactions with a great number of silanol hydroxyl groups, which are generated at the interface owing to the incomplete condensation of alkoxysilanes in sol–gel process.30–32 In fact, up to now, most of the amphiphilic block copolymers which have been successfully used for the preparation mesoporous silica materials contain PEO subchains.15,30,33–40 It is still of interest to explore to employ other block copolymers toward this end.41,42 Recently, Zheng et al.43–45 reported the utilization of several block copolymers which do not contain PEO subchains for the preparation of mesoporous silica materials; the affinity of the subchains of these block copolymer with silica matrix was achieved through the inter-component chemical linkage.43–45In the past years, various amphiphilic block copolymers have been designed and used as the porogens to afford mesoporous silica materials. However, the synthesis of these amphiphilic block copolymers is by no means a trivial task. Depending on type of subchains, different polymerization techniques such as anionic, radical and ring-opening polymerizations must be combined and inter-switched. In addition, proper sequences of different polymerizations must be utilized. If possible, it will be attractive only to utilize homopolymers or random copolymers to access mesoporous silica materials. As well known, nonetheless, a simple utilization of homopolymers (or random copolymers) is not successful since macroscopic phase separation would occur. For instance, Nakanishi et al.46,47 first prepared the macroporous silica monolith with poly(ethylene oxide) (PEO) homopolymer as the porogen. The hydrolysis and condensation of TEOS started from its homogenous mixtures with the PEO samples. With the occurrence of sol–gel reactions, the porogen (viz. PEO) was demixed out and dispersed in the silica gels in the form of the microdomains with micrometer scale. The removal of the segregated PEO microdomains afforded the macroporous silica monoliths. The driving force for the demixing of porogens is the decrease in the contribution of entropy of mixing (ΔSm) to free energy of mixing (ΔGm) owing to formation of silica networks in sol–gel process. Owing to the occurrence of macroscopic phase separation in sol–gel process, the PEO homopolymer was only used for the preparation of macroporous silica materials. If homopolymers can be successfully used as the porogen of mesoporous silica materials, specific measure must be taken to suppress the tendency of macroscopic phase separation. To the best of our knowledge, such an effect remained largely unexplored.
In this work, we explore the use of polystyrene (PS) homopolymers as the porogens to access mesoporous silica materials. The measure taken to suppress the macroscopic phase separation is the functionalization of the end groups of PS homopolymers with tetraethoxysilane (TEOS)-reactive moiety. Toward this end, we first synthesized a series of PS homopolymers with variable molecular weights via atom transfer radical polymerization (ATRP). Thereafter, the single ends of these PS homopolymer chains were functionalized with hydroxyl groups with the combination of substitution and copper(I)-catalyzed Huisgen cycloaddition reaction. The terminal hydroxyl groups are readily reactive with 3-isocyanatopropyl triethoxysilane to afford the PS homopolymers with triethoxysilane ends. The purpose of this work is to elucidate the role of the functionalization of the terminal groups suppressing macroscopic phase separation of PS homopolymers in the sol–gel process and thus the PS homopolymers can be alternatively used as the porogens of mesoporous silica materials. In this work, the mesoporous silica materials would be characterized by means of small angle X-ray scattering (SAXS), transmission electron microscopy (TEM) and Brunauer–Emmett–Teller (BET) measurements.
Experimental
Materials
Styrene (St), sodium azide (NaN3), tetraethoxysilane (TEOS), copper(I) bromide [Cu(I)Br], propargyl alcohol and hydrochloric acid were purchased from Shanghai Reagent Co., China. Before use, St was passed through a alkaline alumina column to remove the inhibitor; propargyl alcohol was distilled under decreased pressure. N,N,N′,N′,N′′-Pentamethyldiethylenetriamine (PMDETA), 3-isocyanatopropyltriethoxysilane (IPTES), methyl 2-bromopropionate and dibutyltin dilaurate (DBTDL) were purchased from Aldrich Co., Shanghai, China. The organic solvents such as N,N′-dimethylformamide (DMF), tetrahydrofuran (THF), methanol and dichloromethane were obtained from Shanghai Reagent Co., China. Before use, THF were refluxed over metal sodium and then distilled.Synthesis of α-hydroxyl-terminated polystyrene homopolymers (PS-OH)
First, α-bromo-terminated polystyrenes (denoted PS-Br) were synthesized via atom transfer radical polymerization (ATRP). Typically, to a 25 mL flask equipped with a magnetic stirrer, styrene (5.000 g, 0.048 mol), methyl 2-bromopropionate (0.417 g, 2.5 mmol), Cu(I)Br (0.359 g, 2.49 mmol), PMDETA (0.432 g, 2.49 mmol) were charged with vigorous stirring. The flask was connected onto a Schleck line to degas via three freeze–evacuate–thaw cycles. The polymerization was carried out at 90 °C for 6 hours to attain a desire conversion of St. After cooling to room temperature, 10 mL of dichloromethane was added to the flask and then the mixture was passed through a neutral alumina column to remove the catalyst. The solution was concentrated via rotary evaporation; the concentrated solution was dropwise added into a large amount of methanol to afford the precipitates. After dried in vacuo at 30 °C for 24 hours, the product (4.770 g) was obtained with the conversion of St to be 95.4%. 1H NMR (CDCl3, ppm): 0.82 [3H, CHCH3] 1.0–1.5 [2H, CH2CH(C6H5)], 1.61–1.93 [1H, CH2CH(C6H5)], 6.24–7.25 [5H, C6H5–], 4.30–4.50 [1H, BrCH(C6H5)CH2], 3.30–3.45 [3H, H3COCO]. GPC: Mn = 4600 Da, with Mw/Mn = 1.07.Second, the above α-bromo-terminated polystyrenes were allowed to react with sodium azide (NaN3) to obtain an α-azido-terminated polystyrenes (denoted PS-N3). Typically, to a flask with a magnetic stirrer, PS-Br (4.000 g, 0.87 mmol, Mn = 4600 Da), N,N′-dimethylformamide (DMF) (2 mL) and NaN3 (2.600 g, 40 mmol) were charged with vigorous stirring. The substitution reaction was performed at room temperature for 24 hours. After the insoluble solids were filtered out, the solution was concentrated via rotary evaporation and then dropwise added into a great amount of methanol to afford the precipitates. After dried in vacuo at 30 °C, the product (3.220 g) was obtained with the yield of 80.5%. 1H NMR (CDCl3, ppm): 0.82 [3H, CHCH3] 1.0–1.5 [2H, CH2CH(C6H5)], 1.61–1.93 [1H, CH2CH(C6H5)], 6.24–7.25 [5H, C6H5–], 3.76–3.98 [1H, –N3CH(C6H5)CH2] and 3.30–3.45 [3H, H3COCO].
Third, the Huisgen 1,3-dipolar cycloaddition of the α-azido-terminated polystyrenes with propargyl alcohol was carried out to afford α-hydroxyl-terminated polystyrenes (denoted PS-OH). Typically, PS-N3 (3.000 g, 0.65 mmol, Mn = 4600 Da), DMF (2 mL) and propargyl alcohol (1.680 g, 30 mmol) were added to a flask with vigorous stirring. This mixture was bubbled with highly pure nitrogen for 30 min and then Cu(I)Br (0.246 g, 1.5 mmol) and PMDETA (0.320 g, 0.15 mmol) were added and then the system was degassed via three freeze–evacuate–thaw cycles. The reaction was carried out at room temperature for 24 hours and the reacted mixture was passed through a neutral alumina column to remove the catalyst. After concentrated via rotary evaporation, the solution was dropwise added into a great amount of methanol to afford the precipitates. After drying in vacuo at 30 °C for 12 hours, the product (2.670 g) was obtained with the yield of 89.0%. 1H NMR (CDCl3, ppm): 0.82 [3H, CHCH3] 1.0–1.5 [2H, CH2CH(C6H5)], 1.61–1.93 [1H, CH2CH(C6H5)], 6.24–7.25 [5H, C6H5–], 4.97–5.08 [1H, N3CH(C6H5)], 4.53–4.68 [2H, –CH2OH] and 3.30–3.45 [3H, H3COCO].
Preparation of mesoporous silica
First, organic–inorganic silica gels were prepared via sol–gel processes. Typically, PS-OH (0.200 g, 0.043 mmol, Mn = 4600 Da), 3-isocyanatopropyl triethoxysilane (IPTES) (27.2 mg, 0.11 mmol), dibutyltin dilaurate (6.3 mg) and 2.5 mL of anhydrous THF were added to a flask containing a magnetic stirrer. This reaction was performed at 55 °C for 6 hours with vigorous stirring. Cooled to room temperature, the reacted mixture was poured into a polyethylene beaker and then TEOS (1.590 g, 7.63 mmol) and THF (3 mL) was added with stirring and then 4 mL of 1.0 M hydrochloric acid (HCl) was added. After vigorous stirring for 30 min, the beaker was sealed to allow the sol–gel process at room temperature for 4 weeks to obtain an organic–inorganic silica gel.Second, the above organic–inorganic silica gel was subjected to pyrolysis at elevated temperature to remove the organic components. Typically, in a tube furnace, the silica gel was heated from room temperature to 450 °C at the heating rate of 10 °C min−1 and then heated up to 550 °C at the heating rate of 5 °C min−1. In an air atmosphere, the gel was maintained at 550 °C for 4 hours to obtain the silica.
Measurement and characterization
Results
Synthesis of α-hydroxyl-terminated polystyrene (PS-OH)
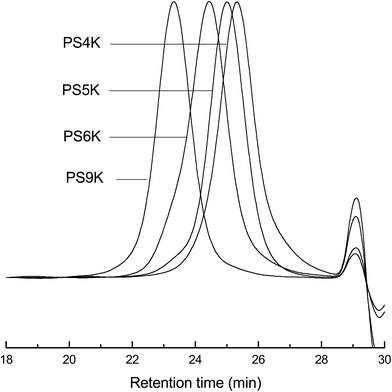
The synthesis route of α-hydroxyl-terminated polystyrene (PS-OH) was shown in Scheme 1. First, α-bromo-terminated polystyrene (PS-Br) was synthesized via the atom transfer radical polymerization of styrene (St) with methyl 2-bromopropionate as the initiator and with the complex of Cu(I)Br with PMDETA as the catalyst. All the PS-Br samples were subjected to gel permeation chromatography (GPC) and the GPC curves are shown in Fig. 1. In all the cases, the unimodal distribution of molecular weights was exhibited and the values of polydispersity were measured to be about Mw/Mn = 1.10. The quite narrow distribution of molecular weights indicates that the PS homopolymers with a series of molecular weights were successfully obtained as summarized in Table 1. Second, the above PS-Br samples were subjected to the reaction with sodium azide (NaN3), allowing the substitution of the terminal bromine atoms with azido groups. The completion of the reactions was monitored by means of nuclear magnetic resonance (NMR) spectroscopy. Finally, the Huisgen 1,3-dipolar cycloaddition of the terminal azido groups of the PS-N3 samples with propargyl alcohol was carried out to obtain the α-hydroxyl-terminated polystyrene (denoted PS-OHs). Representatively shown in Fig. 2 are the 1H NMR spectra of PS5K-Br, PS5K-N3 and PS5K-OH. For PS5K-Br, the signals of resonance at 0.82 and 3.39 ppm are assignable to the protons of the methyl groups from the initiator and those at 1.0–2.5 and 6.0–7.5 ppm are attributable to the protons of methine and methylene groups in the main chain of PS and phenyl groups, respectively. The resonance of methine proton at the chain ends of PS-Br occurred at 4.34 ppm as shown in the enlarged inset. With the occurrence of the substitution reaction, notably, this peak fully shifted to 3.86 ppm whereas other peaks of resonance remained unchanged, indicating that the substitution reaction was undergone completion, i.e., the PS-N3 was successfully obtained. After the PS-N3 was subjected to the Huisgen 1,3-dipolar cycloaddition with propargyl alcohol, there appeared two new signals of resonance at 4.59 and 5.02 ppm, respectively. The former is assignable to the methylene protons of the terminal hydroxymethyl groups whereas the latter to the proton of methine groups connected to triazole ring resulting from the click chemistry. The 1H NMR spectroscopy indicates that the terminal groups of PS-Br samples have been completely converted into the hydroxyl groups. In the FTIR measurements, the complete disappearance of the stretching vibration at 2099 cm−1 assignable to azido groups at the ends of PS5K-N3 samples also confirmed the completion of the click chemistry (the spectra not shown for brevity). The GPC, NMR and FTIR results indicate that the α-hydroxyl-terminated polystyrene (PS-OH) samples were successfully obtained.| Samples | Mn (Da) | Mw/Mn |
|---|---|---|
| PS4K | 3800 | 1.07 |
| PS5K | 4600 | 1.06 |
| PS6K | 5700 | 1.07 |
| PS9K | 8600 | 1.06 |
Preparation of mesoporous silica
The above PS-OH samples were employed as the porogens to prepare the mesoporous silica instead of a variety of surfactants or block copolymers via sol–gel process.11–15 To increase the affinity of the PS homopolymers with silica matrix, the PS-OH samples were reacted with 3-isocyanatopropyltriethoxysilane (IPTES) to endow the PS homopolymers with the reactivity of tetraethoxysilane (TEOS) via the terminal triethoxysilane moieties (see Scheme 2). Thereafter, the PS homopolymers terminated with triethoxysilane moieties were incorporated into TEOS to perform the sol–gel reaction to afford the organic–inorganic silica gels. Shown in Fig. 3 are the FTIR spectra of PS-OH, IPTES and their equimolar (with respect of isocyanate groups of IPTES) reacted product. For PS-OH, the terminal hydroxyl groups were characteristic of the band at 3430 cm−1, attributable to the stretching vibration of hydroxyl groups. For IPTES, the intense band at 2274 cm−1 is assignable to the stretching vibration of isocyanate (–NCO) groups. Upon adding IPTES to PS-OH, notably, the band at 2274 cm−1 totally disappeared, indicating that all the NCO groups reacted with the terminal hydroxyl groups of PS-OH. Concurrently, there appeared two new bands at 1655 and 1076 cm−1, respectively. The former is assignable to the stretching vibration of carbamate (–NHCOO–) moiety whereas the latter resulted from the Si–O bonds in triethoxysilane moiety. The appearance of these two bands indicates that the PS-OH homopolymers were successfully reacted with IPTES. Thereafter, the IPTES-functionalized PS samples were incorporated into TEOS to prepare the organic–inorganic silica gels via sol–gel processes. The compositions of the silica gels were summarized into Table 2. Representatively shown in Fig. 3 is the FTIR spectrum of Gel_PS5K_12.5. It is seen that the silica gel exhibited the spectral bands from PS at 3200–2800, 1520–1390 and 700 cm−1 and from silica at 1072 cm−1. The FTIR spectroscopy indicates that the organic–inorganic silica gels were successfully obtained. | ||
| Fig. 3 FTIR spectra of PS-OH (Mn = 4600 Da), IPTES, PS-IPTES (Mn = 4600 Da), Gel_PS5K_12.5 and Silica_PS5K_12.5. | ||
| Mesoporous silica | Organic–inorganic silica gels | [PS] (wt%) | Vpore (cm3 g−1) | SBET (m2 STP per g) |
|---|---|---|---|---|
| Silica_PS5K_5 | GEL_PS5K5TEOS95 | 5 | 0.177 | 402 |
| Silica_PS5K_10 | GEL_PS5K10TEOS90 | 10 | 0.162 | 381 |
| Silica_PS5K_12.5 | GEL_PS5K12.5TEOS87.5 | 12.5 | 0.144 | 487 |
| Silica_PS5K_15 | GEL_PS5K15TEOS85 | 15 | 0.101 | 306 |
| Silica_PS5K_20 | GEL_PS5K20TEOS80 | 20 | 0.204 | 560 |
| Silica_P4K_12.5 | GEL_PS4K12.5TEOS87.5 | 12.5 | 0.126 | 340 |
| Silica_PS5K_12.5 | GEL_PS5K12.5TEOS87.5 | 12.5 | 0.144 | 487 |
| Silica_PS6K_12.5 | GEL_PS6K12.5TEOS87.5 | 12.5 | 0.243 | 762 |
| Silica_PS9K_12.5 | GEL_PS9K12.5TEOS87.5 | 12.5 | 0.255 | 756 |
The above organic–inorganic silica gels were used as the precursors to obtain the mesoporous silica via the removal of the organic component (viz. PS) with pyrolysis at elevated temperatures. To determine the temperatures degrading the organic components, the organic–inorganic silica gels were first subjected to thermogravimetric analysis (TGA) and the TGA profiles were shown in Fig. 4. It is seen that these silica gels displayed the initial degradation at 320–390 °C, depending on the contents of PS. The initial degradation temperatures (Td) decreased with increasing the contents of PS. The initial mass loss was mainly responsible for the degradation of the PS microdomains in the organic–inorganic silica gels. Notably, the thermal decomposition was continued to about 500 °C and thereafter the mass no longer decreased. The appearance of the degradation plateaus indicates that the organic components in these gels were completely decomposed, i.e., the organic–inorganic silica gels were converted into the inorganic silica. The yields of silica were measured to be 76.4, 70.3, 67.7, 63.4 and 58.7% for the organic–inorganic silica gels containing 5, 10, 12.5, 15 and 20 wt% of IPTES-functionalized PS (Mn = 4600 Da). The values of silica yields were quite close to those calculated according to the feed ratio, suggesting that the organic components were fully decomposed. The complete removal of the organic components was also evidenced by the observation that all the FTIR bands assignable to PS completely disappeared (see Fig. 3). In views of the results of TGA, the conditions of pyrolysis were thus determined as depicted in the section of Experimental. According the condition, all the organic–inorganic silica gels were subjected to pyrolysis in a tube furnace to obtain the porous silica materials.
Morphologies of mesoporous silica
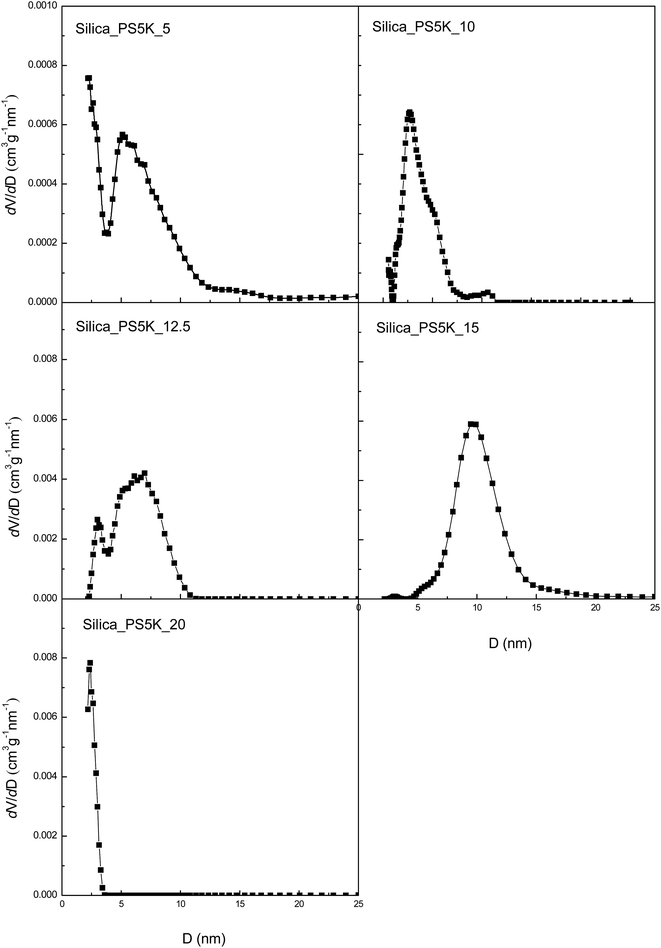
The above silica materials were subjected to the morphological observation by means of transmission electron microscopy (TEM). Shown in Fig. 5 are the TEM images of the silica materials prepared from the PS homopolymer with Mn = 4600 Da. It is seen that the silica materials displayed the nanoporous structures. The quantity of the nanopores increased with increasing the content of the PS homopolymer; the size and shape of the nanopores were quite dependent on the content of the PS homopolymer in the gels. For Silica_PS5K_5, the spherical nanopores with the size of 5–10 nm in diameter were dispersed in the continuous silica matrix. With increasing the content of PS5K, the size of the nanopores increased. The spherical nanopores in Silica_PS5K_10 had the size as large as 20–30 nm. Further increasing the content of PS5K, the size of the nanopores decreased whereas the quantity of the nanopores increased. For Silica_PS5K_12.5, the size of the pores was decreased to about 10 nm in diameter. While the content of PS5K in the organic–inorganic silica gel was 15 wt%, the spherical nanopores were fully transformed into the cylindrical nanopores with the diameter of cross section to be ca. 10 nm. The formation of the nanopores in the silicas was further evidenced by the measurement of specific surface area with nitrogen sorption experiments. The nitrogen sorption isotherms of the mesoporous silica materials are shown in Fig. 6; the structural information derived from the nitrogen adsorption data was summarized in Table 2. It is noted that the mesoporous silica materials exhibited typical type-IV sorption isotherms with H2-type hysteresis loops. This observation suggests that the silica materials had the cage-like nanopores with small windows (i.e., micropores). For Silica_PS5K_5, Silica_PS5K_10, Silica_PS5K_12.5, Silica_PS5K_15 and Silica_PS5K_20, the Brunauer–Emmett–Teller (BET) surface areas were measured to be 402, 381, 487, 306 and 560 m2 g−1, respectively, the pore volumes ranging from 0.10 to 0.20 cm3 g−1. Shown in Fig. 7 are the plots of dV/dD as functions of the pore width (D) for these mesoporous silica materials. For all the received silica materials, the broad distribution of pore width was exhibited. Notably, there were some micropores with the size of 2–4 nm; there were also the mesopores with the width of 10–40 nm in the porous silica materials. It is proposed that the former (viz. micropores) mainly resulted from the removal of the organic moiety from the reaction of the end groups of the PS samples with IPTES whereas the latter from the removal of the PS microdomains as observed by means of TEM.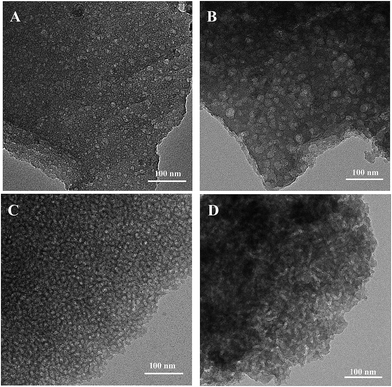 | ||
| Fig. 5 TEM micrographs of the mesoporous silica materials: (A) Silica_PS5K_5; (B) Silica_PS5K_10; (C) Silica_PS5K_12.5 and (D) Silica_PS5K_15. | ||
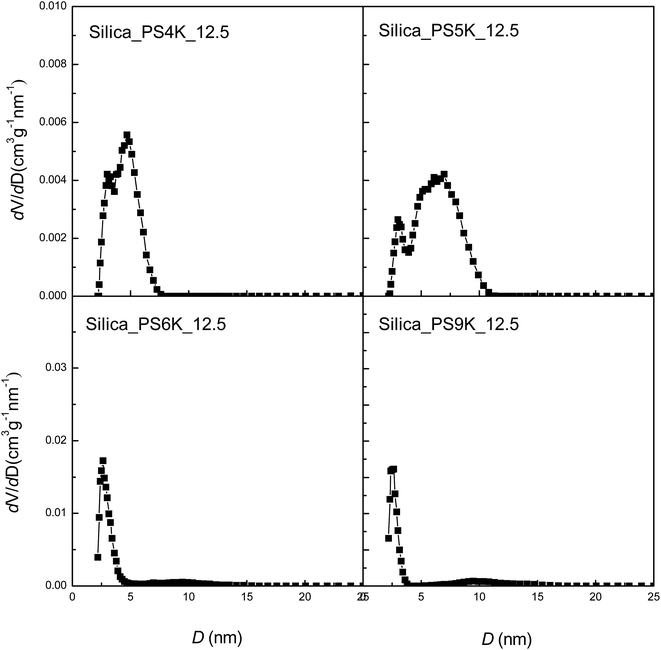
The formation of the mesoporous structures reminded examining the effect of molecular weights of PS homopolymers on the porosity of the mesoporous silica materials. Shown in Fig. 8 are the TEM micrographs of the silica materials resulting from the organic–inorganic silica gels containing 12.5 wt% of the PS homopolymers with variable molecular weights. In all the cases, the spherical nanopores were exhibited with the size of ca. 20 nm in diameter. Notably, the size of the nanopores slightly decreased with increasing the molecular weights of the PS homopolymers. The formation of the nanoporous silica materials was also evidenced by the measurements of specific surface areas with nitrogen sorption experiments as shown in Fig. 9. All these mesoporous silica materials exhibited typical type-IV sorption isotherms with H2-type hysteresis loops. The BET measurements indicate that the silica materials possessed the cage-like nanopores with small windows (i.e., micropores) in the walls. It is noted that the specific surface areas increased with the molecular weights (see Table 2). This observation suggests that the size of the mesopores slightly decreased with increasing the molecular weight of PS homopolymers, which was in good agreement with the TEM results. With the identical content of PS samples (viz. 12.5 wt%), notably, the pore volume slightly increased with increasing the molecular weights of the PS samples. This observation can be interpreted on the basis of the extent of microphase separation of PS out of the silica matrices, which was quite dependent on the molecular weights of PS. In principle, the higher the molecular weights of PS the higher the extent of microphase separation. Shown in Fig. 10 are the plots of dV/dD as functions of the pore width (D). The broad size distribution from micropores to mesopores was also displayed for all the received silica materials.
Discussion
All the organic–inorganic silica gels containing the PS homopolymers with the functionalization of the terminal groups were homogenous and transparent, suggesting that no macroscopic phase separation occurred in the sol–gel process. This observation was in marked contrast to the case that the PS homopolymers were directly employed without the functionalization of the terminal groups. The transparency suggests that the PS homopolymers existed in the form of the microdomains with the scale lower than the wavelength of visible light. To prove this speculation, all the organic–inorganic silica gels were subjected to small angle X-ray scattering (SAXS) and the SAXS profiles are presented in Fig. 11. For comparison, the SAXS profiles of the corresponding mesoporous silica materials are shown in Fig. 12. For the silica gels, the scattering phenomena were exhibited in all the cases, indicating that the organic–inorganic silica gels were indeed microphase-separated, i.e., the PS microdomains were demixed out of the silica matrices. For the mesoporous silicas, the scattering profiles were also displayed, indicating the formation of the mesoporous structures. The results of SAXS were in good agreement with those of TEM. More importantly, the SAXS profiles of the mesoporous silica materials were quite similar to those of the organic–inorganic silica gels, suggesting that the pyrolysis did not alter the backbones of the organic–inorganic silica gels, i.e., the formation of the nanopores in the silica materials resulted from the removal of the PS microdomains in the organic–inorganic silica gels. Compared to the silica gels, the primary scattering peaks of the mesoporous silicas shifted to the positions with the lower scatter vector (qm) values. The shift is attributable to the shrinkage of the silica gels due to the removal of the organic component (viz. PS) together with the further condensation of the silica gels at elevated temperature. | ||
| Fig. 11 SAXS profiles of the organic–inorganic silica gels by the use of the PS homopolymer with Mn = 4600 Da. | ||
 | ||
| Fig. 12 SAXS profiles of the mesoporous silica materials from the organic–inorganic silica gels containing the PS homopolymer with Mn = 4600 Da. | ||
It should be pointed out all the PS homopolymers can be readily dissolved in the precursor of silica (viz. TEOS). This observation suggests that the sol–gel process of TEOS in the presence of PS homopolymers started from the homogenous mixtures. It is plausible to propose that the formation of PS microdomains in the organic–inorganic silica gels followed the reaction-induced phase separation (RIPS) mechanism.48–50 From the thermodynamic viewpoint, the driving force for RIPS is from the decrease in the contribution of entropy of mixing (ΔSm) to free energy of mixing (ΔGm) owing to the formation of the silica networks.51 From the kinetic viewpoint, reaction-induced phase separation mainly follows Spinodal decomposition mechanism.48–50 Generally, reaction-induced phase separation occurred at the micrometer scale while homopolymers (or random copolymers) were used.48–50 As a consequence, the removal of the labile phases in the phase-separated silica gels would afford the macroscopic pores with the size on the scale of micrometer. This approach has been applied to prepare macroporous silica materials.47 To avoid the occurrence of macroscopic phase separation, generally, amphiphilic molecules (viz. low molecular surfactants and block copolymers) must be used instead of homopolymers. Under this circumstance, the microphase-separated morphologies are formed at the scale of nanometer; the removal of the nanodomains in the silica gels would afford the mesopores in the received silica materials. In ample literature, a variety of amphiphilic molecules such as ionic and nonionic surfactants, block copolymers have been applied to prepare mesoporous silica materials. In general, these amphiphiles are composed of both silica-philic and silica-phobic components.39,51–54
In the present case, macroscopic phase separation did not occur in the organic–inorganic silica gels in the composition investigated although the PS homopolymers were employed instead of amphiphilic molecules. An important difference between the PS samples and traditional homopolymers is that the single ends of all these PS homopolymers were functionalized with triethoxysilane moieties. It is proposed that the functionalization of the chain ends played the crucial role suppressing the occurrence of the macroscopic phase separation. In the present case, the single end groups of the PS chains (viz. triethoxysilane groups) were capable of reacting with TEOS in the sol–gel process. As the sol–gel process proceeded, the PS chains were in situ transformed into the new amphiphiles composed of PS chains and silica component. As a consequence, the PS chains were grafted onto silica networks through Si–O–Si linkages. The inter-component reaction between the single ends of the PS homopolymers and TEOS confined the reaction-induced phase separation of PS on the nanometer scale, i.e., the PS microdomains (or nanodomains) with spherical or cylindrical morphologies were formed in the organic–inorganic silica gels. The removal of the PS microdomains finally afforded the nanopores in the silica materials as depicted in Scheme 2. It should be pointed out that the microdomains formed in the process of reaction-induced microphase separation were quite different from those formed via the self-assembly approach of those well-defined amphiphiles in TEOS as rehearsed in ample literature. In the self-assembly approach of well-defined amphiphiles, the morphologies of labile microdomains were controlled by the equilibrium thermodynamics between silica-phobic and silica-philic portions in the mixtures of the amphiphiles with TEOS. In contrast, the competitive kinetics between microphase separation and sol–gel reaction would be involved with the mixing system of reaction-induced phase separation except for the above-mentioned thermodynamic factors. The morphologies of the resulting materials are quite dependent on the relative rates of the polymerization and the phase separation. It is known that the rates of polymerization and phase separation are significantly influenced by the viscosity of the reactive systems in the process of in situ polymerization.48–50 It is expected that high viscosity resulting from fast polymerization would hinder the formation of the segregated microdomains with big sizes. In the present case, the initial viscosity of the mixtures was mainly determined by the contents of the PS homopolymers and the molecular weights of the PS homopolymers. The viscosity of the initial mixtures increased with the contents of the PS homopolymers. This speculation has been confirmed by the observation that the sizes of the pores decreased with increasing the content of PS5K in the organic–inorganic silica gels (see Fig. 5). It should be pointed out that the cylindrical (or worm-like) microdomains in the silica gels could be formed via the interconnection of the globule microdomains. With the identical content of PS homopolymers (12.5 wt%), the larger the viscosity of the mixtures the higher the molecular weights of the PS homopolymers. As a result, the size of PS microdomains decreased with increasing the molecular weights of PS homopolymers owing to the increased viscosity. In fact, we indeed observed that the size of the spherical nanopores decreased with increasing the molecular weights of PS samples (see Fig. 8). Notably, the pore volume slightly increased with increasing the molecular weights of the PS homopolymers while the contents of the PS homopolymers were 12.5% in the organic–inorganic gels (see Table 2). It is proposed that the measured values of pore volumes could be affected by the following factors: (i) the degree of phase separation and (ii) the accessibility of the pores in the silica materials. It is known that the degree of phase separation is quite dependent on the molecular weights of component polymers in polymer blends; the higher the molecular weights of PS the higher the extent of microphase separation.51 It is plausible to propose that in the present case, the demixing degree of PS out of the silica matrices were quite dependent on the molecular weights of the PS homopolymers. In the organic–inorganic silica gels the PS samples with the lower molecular weights (e.g., PS4K and PS5K) could be trapped in the silica matrices at the segmental level. The pyrolysis of this portion of PS chains would not yield the mesopores. It is plausible to propose that the values of the pore volumes are only attributable to those pores which are accessible with nitrogen sorption measurements and do not reflect all the free volumes in the received silica materials.
Conclusions
A series of PS homopolymers with various molecular weights were synthesized via atom transfer radical polymerization (ATRP). The single ends of these PS homopolymers were further functionalized with hydroxyl groups via copper(I)-catalyzed cycloaddition reaction. The hydroxyl-functionalized PS homopolymers were capable of reacting with 3-isocyanatopropyltriethoxysilane (IPTES) to afford triethoxysilane-terminated PS homopolymers. The sol–gel process of TEOS was carried out in the presence of the triethoxysilane-terminated PS homopolymers to afford a series of organic–inorganic silica gels. The SAXS results showed that the organic–inorganic silica gels were microphase-separated. The organic–inorganic silica gels were successfully employed as the precursors to prepare the mesoporous silica materials via the removal of the PS microdomains with pyrolysis at elevated temperatures. Transmission electron microscopy (TEM) showed that all the silica materials displayed the spherical or cylindrical nanopores with the size of the nanopores of 10–30 nm. The mesoporosity was further confirmed by the measurement of specific surface areas by nitrogen sorption experiments. The specific surface areas can be modulated in terms of the contents and molecular weights of PS homopolymers. It is proposed that the functionalization of chain ends for the PS homopolymers with triethoxysilane groups resulted that the reaction-induced phase separation of PS homopolymers was confined on the nanometer scale. This approach is in marked contrast to the formation of the microdomains in silica gels with the utilization of amphiphiles via self-assembly approach.Acknowledgements
The financial supports from Natural Science Foundation of China (No. 51133003, 21274091 and 21304058) were gratefully acknowledged. The authors thank the Shanghai Synchrotron Radiation Facility for the support under the projects of No. 10sr0260 & 10sr0126.References
- C. J. Brinker and G. W. Scherer, Sol-gel Science, Academic, New York, 1990 Search PubMed
.
- A. Corma, Chem. Rev., 1997, 97, 2373–2420 CrossRef CAS PubMed
.
- P. Yang, D. Zhao, D. I. Margolese, B. F. Chmelka and G. D. Stucky, Nature, 1998, 396, 152–155 CrossRef CAS
.
- Y. Zhu, J. Shi, W. Shen, X. Dong, J. Feng, M. Ruan and Y. Li, Angew. Chem., 2005, 117, 5213–5217 CrossRef
.
- B. Tian, H. Yang, X. Liu, S. Xie, C. Yu, J. Fan, B. Tu and D. Zhao, Chem. Commun., 2002, 1824–1825 RSC
.
- A. Stein, Adv. Mater., 2003, 15, 763–775 CrossRef CAS
.
- M. Kruk, Acc. Chem. Res., 2012, 45, 1678–1687 CrossRef CAS PubMed
.
- M. Kruk, B. Dufour, E. B. Celer, T. Kowalewski, M. Jaroniec and K. Matyjaszewski, Chem. Mater., 2006, 18, 1417–1424 CrossRef CAS
.
- D. Kuang, T. Brezesinski and B. Smarsly, J. Am. Chem. Soc., 2004, 126, 10534–10535 CrossRef CAS PubMed
.
- T. H. Nguyen, M. n. Vayer, D. Grosso, H. Amenitsch and C. Sinturel, J. Phys. Chem. C, 2012, 116, 5295–5302 CAS
.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed
.
- J. M. Kim and G. D. Stucky, Chem. Commun., 2000, 1159–1160 RSC
.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS
.
- X. Liu, B. Tian, C. Yu, F. Gao, S. Xie, B. Tu, R. Che, L. M. Peng and D. Zhao, Angew. Chem., Int. Ed., 2002, 41, 3876–3878 CrossRef CAS
.
- C. Yu, Y. Yu and D. Zhao, Chem. Commun., 2000, 575–576 RSC
.
- S. Che, Z. Liu, T. Ohsuna, K. Sakamoto, O. Terasaki and T. Tatsumi, Nature, 2004, 429, 281–284 CrossRef CAS PubMed
.
- H. Jin, Z. Liu, T. Ohsuna, O. Terasaki, Y. Inoue, K. Sakamoto, T. Nakanishi, K. Ariga and S. Che, Adv. Mater., 2006, 18, 593–596 CrossRef CAS
.
- C. Gao, H. Qiu, W. Zeng, Y. Sakamoto, O. Terasaki, K. Sakamoto, Q. Chen and S. Che, Chem. Mater., 2006, 18, 3904–3914 CrossRef CAS
.
- M. Imperor-Clerc, P. Davidson and A. Davidson, J. Am. Chem. Soc., 2000, 122, 11925–11933 CrossRef CAS
.
- X. Wang, K. S. Lin, J. C. Chan and S. Cheng, J. Phys. Chem. B, 2005, 109, 1763–1769 CrossRef CAS PubMed
.
- A. Sayari, B.-H. Han and Y. Yang, J. Am. Chem. Soc., 2004, 126, 14348–14349 CrossRef CAS PubMed
.
- R. Ryoo, C. H. Ko, M. Kruk, V. Antochshuk and M. Jaroniec, J. Phys. Chem. B, 2000, 104, 11465–11471 CrossRef CAS
.
- D. Grosso, A. Balkenende, P. Albouy, A. Ayral, H. Amenitsch and F. Babonneau, Chem. Mater., 2001, 13, 1848–1856 CrossRef CAS
.
- P. Schmidt-Winkel, W. W. Lukens, D. Zhao, P. Yang, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1999, 121, 254–255 CrossRef CAS
.
- Y. Han, S. S. Lee and J. Y. Ying, Chem. Mater., 2006, 18, 643–649 CrossRef CAS
.
- H. Zhang, J. Sun, D. Ma, X. Bao, A. Klein-Hoffmann, G. Weinberg, D. Su and R. Schlögl, J. Am. Chem. Soc., 2004, 126, 7440–7441 CrossRef CAS PubMed
.
- M. Kruk and L. Cao, Langmuir, 2007, 23, 7247–7254 CrossRef CAS PubMed
.
- D. ShengáSu, Chem. Commun., 2005, 5343–5345 Search PubMed
.
- A. Katiyar, S. Yadav, P. G. Smirniotis and N. G. Pinto, J. Chromatogr. A, 2006, 1122, 13–20 CrossRef CAS PubMed
.
- S. Lu, M. M. Melo, J. Zhao, E. M. Pearce and T. Kwei, Macromolecules, 1995, 28, 4908–4913 CrossRef CAS
.
- J.-G. Li, W.-C. Chen and S.-W. Kuo, Microporous Mesoporous Mater., 2012, 163, 34–41 CrossRef CAS
.
- R. Takahashi, K. Nakanishi and N. Soga, J. Sol-Gel Sci. Technol., 2000, 17, 7–18 CrossRef CAS
.
- M. Templin, A. Franck, A. Du Chesne, H. Leist, Y. Zhang, R. Ulrich, V. Schädler and U. Wiesner, Science, 1997, 278, 1795–1798 CrossRef CAS PubMed
.
- Y. Deng, T. Yu, Y. Wan, Y. Shi, Y. Meng, D. Gu, L. Zhang, Y. Huang, C. Liu and X. Wu, J. Am. Chem. Soc., 2007, 129, 1690–1697 CrossRef CAS PubMed
.
- K. Yu, A. J. Hurd, A. Eisenberg and C. J. Brinker, Langmuir, 2001, 17, 7961–7965 CrossRef CAS
.
- B. Smarsly, G. Xomeritakis, K. Yu, N. Liu, H. Fan, R. A. Assink, C. A. Drewien, W. Ruland and C. J. Brinker, Langmuir, 2003, 19, 7295–7301 CrossRef CAS
.
- K. Yu, B. Smarsly and C. J. Brinker, Adv. Funct. Mater., 2003, 13, 47–52 CrossRef CAS
.
- J. Wei, Y. Deng, J. Zhang, Z. Sun, B. Tu and D. Zhao, Solid State Sci., 2011, 13, 784–792 CrossRef CAS
.
- J. Zhang, Y. Deng, J. Wei, Z. Sun, D. Gu, H. Bongard, C. Liu, H. Wu, B. Tu and F. Schüth, Chem. Mater., 2009, 21, 3996–4005 CrossRef CAS
.
- J. G. Li, R. B. Lin and S. W. Kuo, Macromol. Rapid Commun., 2012, 33, 678–682 CrossRef CAS PubMed
.
- D. Niu, Z. Ma, Y. Li and J. Shi, J. Am. Chem. Soc., 2010, 132, 15144–15147 CrossRef CAS PubMed
.
- S. Wang, M. Zhang, D. Wang, W. Zhang and S. Liu, Microporous Mesoporous Mater., 2011, 139, 1–7 CrossRef CAS
.
- W. Xun, Y. Yi, C. Zhang and S. Zheng, J. Macromol. Sci., Part A: Pure Appl. Chem., 2013, 50, 399–410 CrossRef CAS
.
- R. Yu and S. Zheng, Ind. Eng. Chem. Res., 2015, 54, 6454–6466 CrossRef CAS
.
- M. Huang, L. Li and S. Zheng, Microporous Mesoporous Mater., 2016, 225, 9–25 CrossRef CAS
.
- K. Nakanishi, H. Komura, R. Takahashi and N. Soga, Bull. Chem. Soc. Jpn., 1994, 67, 1327–1335 CrossRef CAS
.
- K. Nakanishi and N. Soga, J. Am. Ceram. Soc., 1991, 74, 2518–2530 CrossRef CAS
.
- K. Yamanaka, Y. Takagi and T. Inoue, Polymer, 1989, 30, 1839–1844 CrossRef CAS
.
- T. Inoue, Prog. Polym. Sci., 1995, 20, 119–153 CrossRef CAS
.
- R. J. Williams, B. A. Rozenberg and J.-P. Pascault, in Polymer Analysis Polymer Physics, Springer, 1997, pp. 95–156 Search PubMed
.
- P. J. Flory, Principles of Polymer Chemistry, Cornell University Press, Ithaca, NY, 1953 Search PubMed
.
- D. Chen, Z. Li, C. Yu, Y. Shi, Z. Zhang, B. Tu and D. Zhao, Chem. Mater., 2005, 17, 3228–3234 CrossRef CAS
.
- P. Schmidt-Winkel, W. W. Lukens, P. Yang, D. I. Margolese, J. S. Lettow, J. Y. Ying and G. D. Stucky, Chem. Mater., 2000, 12, 686–696 CrossRef CAS
.
- G.-R. Yi, J. H. Moon and S.-M. Yang, Chem. Mater., 2001, 13, 2613–2618 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |