Green process development for the preparation of 2,6-dibromo-4-nitroaniline from 4-nitroaniline using bromide–bromate salts in an aqueous acidic medium†
Abstract
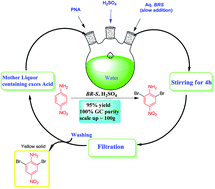
An organic solvent-free process for the preparation of 2,6-dibromo-4-nitroaniline, (an important intermediate in the synthesis of azo disperse dyes) from 4-nitroaniline using 2 : 1 bromide–bromate salts under an aqueous acidic medium at ambient conditions has been developed. The 2 : 1 bromide–bromate couple could be obtained by mixing pure NaBr/NaBrO3 salts or by adjusting the 5 : 1 mole ratio of NaBr/NaBrO3 (obtained in an aqueous solution as an intermediate from the bromine manufacture industry by a cold process) to 2 : 1 by the addition of a suitable oxidant. After completion of the reaction the product was purified by simple filtration and washing with water. The aqueous acidic filtrate was recycled up to five times without loss of purity and yield of the product. The method was extended to other aromatic substrates.
- This article is part of the themed collection: Editors Collection for RSC Advances - India

 Please wait while we load your content...
Please wait while we load your content...