Influence of Ho3+ doping on the temperature sensing behavior of Er3+–Yb3+ doped La2CaZnO5 phosphor
Vijay Kumarab,
S. Som bc,
S. Duttab,
Subrata Das*c and
H. C. Swart*b
bc,
S. Duttab,
Subrata Das*c and
H. C. Swart*b
aDepartment of Applied Physics, Chandigarh University, Gharuan, Mohali, Punjab-140413, India
bDepartment of Physics, University of the Free State, Box 339, Bloemfontein 9300, South Africa. E-mail: swarthc@ufs.ac.za; Fax: +27-58-718-444; Tel: +27-514013852 Tel: +27-58-718-5308
cDepartment of Chemical Engineering, National Taiwan University, Taipei, 10617, Taiwan. E-mail: phy_subrata@yahoo.co.in
First published on 2nd September 2016
Abstract
In this paper, a series of Er3+/Yb3+ and Er3+/Yb3+/Ho3+ codoped La2CaZnO5 (LCZ) upconversion (UC) phosphors were synthesized by the combustion route. The UC emission from LCZ phosphors, codoped with fixed Er3+, Ho3+, and various Yb3+ concentrations has been investigated. The structural and upconversion properties of the synthesized phosphors were studied in detail. Under 980 nm laser excitation, the codoped samples showed green UC emission that consisted of three well-known emission bands centered at 522, 548 and 672 nm generated by the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+ ions, respectively. The emission intensities of these bands have been enhanced sufficiently on codoping of Yb3+ ions in the LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+ system. An effort has been presented to explain the enhancement on the basis of a power dependence study and an energy level diagram. The luminescence lifetimes of the green emission of the LCZ samples with different codoping were also recorded and incorporated to explain the energy transfer mechanism. The strong temperature dependence of the fluorescent intensity ratio between two green emissions makes the material suitable for temperature sensing purposes and it is also suitable up to high temperatures of 500 K. A relatively high-temperature sensor with good sensitivity 0.00625 K−1 was found from the observed results. An increment of 6% for the sensitivity is observed over the existing LCZ phosphor after co-doping with Ho3+. These results indicate that the Er3+/Ho3+/Yb3+ codoped LCZ material is an effective UC phosphor and may be a potential candidate for high-temperature sensors.
Er3+ system. An effort has been presented to explain the enhancement on the basis of a power dependence study and an energy level diagram. The luminescence lifetimes of the green emission of the LCZ samples with different codoping were also recorded and incorporated to explain the energy transfer mechanism. The strong temperature dependence of the fluorescent intensity ratio between two green emissions makes the material suitable for temperature sensing purposes and it is also suitable up to high temperatures of 500 K. A relatively high-temperature sensor with good sensitivity 0.00625 K−1 was found from the observed results. An increment of 6% for the sensitivity is observed over the existing LCZ phosphor after co-doping with Ho3+. These results indicate that the Er3+/Ho3+/Yb3+ codoped LCZ material is an effective UC phosphor and may be a potential candidate for high-temperature sensors.
Introduction
Rare earth (RE) ion-doped upconversion (UC) materials have been the subject of scientific interest due to their significant applications in a variety of fields such as, display devices, temperature sensors, solar cell, bio-imaging, optoelectronics devices, finger print detection, etc.1–6 Among all these applications, RE-doped UC phosphor based temperature sensors are drawing much attention from the scientific community.7,8 Traditional temperature sensors are based on a liquid and metal expansion principle, and measure the temperature during the heat flow using an invasive probe.9 Though this strategy suffers from some limitations, including spatial resolution, sensitivity and accuracy of detection. This method is also not applicable in many places, such as corrosive environments, coal mines, refineries, etc.10–12 To overcome these problems, temperature sensing based on a non-contact temperature measurement technique such as infrared (IR) thermometry, Raman spectroscopy, and luminescence can be implemented.10,11 One practical approach to measuring the temperature of RE-doped UC materials is the fluorescence intensity ratio (FIR) technique. This method is based on the temperature connection of the FIR between two thermally coupled levels of RE ions as a function of external temperature.10–12 FIR, in context, is of particular importance for better accuracy, high sensitivity and independence of the measurement conditions.9–12In the last decades, attention has been attracted towards the ternary oxides RE2MZO5 (RE = rare earth, M = Ba, Ca, Z = Cu, Zn) based UC and down-conversion materials.13 RE2MZO5 based hosts, in context, are of certain significance due to their excellent structural, physical, chemical, magnetic, optical and superconducting properties. The luminescence properties of La2BaZnO5 and Gd2BaZnO5 activated with Eu3+, Tb3+ and Tm3+ were reported for the first time in the year of 1985.13g Thereafter, significant research efforts have been directed towards the luminescent properties of RE doped BaRE2ZnO5 phosphors synthesized by various methods.13h,i The optical and luminescent properties of La2CaZnO5 host doped with different RE ions have been investigated mainly for down-conversion.14
The Yb3+ ion was demonstrated to be an attractive sensitizer for RE3+ ions in various hosts with not only the improved luminescent intensity but also widened excitation spectrum.2 There are some well-known combinations of the photon energies Er3+–Yb3+, Ho3+–Yb3+, and Tm3+–Yb3+. These combinations were extensively studied by various authors in a significant number of hosts due to their potentiality conversion of the NIR light into visible light. The UC properties of the RE doped systems La2BaZnO5 and Gd2BaZnO5 have been investigated by Birkel and coworkers.15 Recent results suggest that the sol–gel derived La2CaZnO5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+–Yb3+ phosphor is an efficient green UC phosphor.16 They have also studied optical thermometry in the temperature scale of 298–513 K adopting the FIR technique. But, the internal heating in the material and optical heating generated by the laser excitation has only been rarely described.17a It is worth mentioning that, the interaction between the electron and phonon could be improved by the effect of quantum confinement among phonons, which results in internal heat generation in crystalline phosphors. Therefore, internal heating must be prominent for nanocrystalline solids.17b However, the optical heating is related to the interaction between phosphors and the excitation source. When any phosphor material is exposed to the laser irradiation, some portion of the absorbed photons in the phosphor is transformed into heat energy via nonradiative processes owing to which the material gets heated optically.17c The nano thermometry behaviour by using the green emissions from Er3+ ions in the Er3+–Ho3+–Yb3+ triply doped La2CaZnO5 (LCZ) phosphors upon NIR excitation have not been investigated at this stage. Moreover, recently, there is a rising interest in the development of temperature sensing technique that is based on the utilization of fluorescent nanomaterials or nanoparticles.18,19 Vetrone et al.20 reported that the fluorescent NaYF4
Er3+–Yb3+ phosphor is an efficient green UC phosphor.16 They have also studied optical thermometry in the temperature scale of 298–513 K adopting the FIR technique. But, the internal heating in the material and optical heating generated by the laser excitation has only been rarely described.17a It is worth mentioning that, the interaction between the electron and phonon could be improved by the effect of quantum confinement among phonons, which results in internal heat generation in crystalline phosphors. Therefore, internal heating must be prominent for nanocrystalline solids.17b However, the optical heating is related to the interaction between phosphors and the excitation source. When any phosphor material is exposed to the laser irradiation, some portion of the absorbed photons in the phosphor is transformed into heat energy via nonradiative processes owing to which the material gets heated optically.17c The nano thermometry behaviour by using the green emissions from Er3+ ions in the Er3+–Ho3+–Yb3+ triply doped La2CaZnO5 (LCZ) phosphors upon NIR excitation have not been investigated at this stage. Moreover, recently, there is a rising interest in the development of temperature sensing technique that is based on the utilization of fluorescent nanomaterials or nanoparticles.18,19 Vetrone et al.20 reported that the fluorescent NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Yb3+ nanoparticles can be utilized as nanothermometers. They have further stated that the ratio between the green emissions bands of the Er3+ ion offers an optical system that in terms regulate temperature distributions in liquids using confocal fluorescence. This opens up the possibility of creating RE3+ ions doped UC nanomaterials that can act as thermal probes with interesting applications in biosensors, fluorescent imaging, and therapeutic purposes, etc.18–20
Er3+, Yb3+ nanoparticles can be utilized as nanothermometers. They have further stated that the ratio between the green emissions bands of the Er3+ ion offers an optical system that in terms regulate temperature distributions in liquids using confocal fluorescence. This opens up the possibility of creating RE3+ ions doped UC nanomaterials that can act as thermal probes with interesting applications in biosensors, fluorescent imaging, and therapeutic purposes, etc.18–20
In this work, we have focused on the La2CaZnO5 host doped with lanthanide ions (Yb3+, Ho3+, Er3+) for strong green and red UC emission properties. An attempt has been made to increase the UC and optical heating performance of the previously reported Yb3+, Er3+ codoped material for optical temperature sensing by changing the synthesis condition and the addition of another co-dopant.16 In this paper, we produced LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Ho3+/Yb3+ UC phosphors via solution combustion method. Control of crystallization in the phosphors was studied by X-ray diffraction (XRD). The UC emission characteristics of the synthesized materials have been studied upon 980 nm diode laser excitation at room temperature. The effect of doping and the mechanism involved in the UC process has been studied in detail. The color of the infrared emissions of the codoped sample was also tuned by varying the laser power. Meanwhile, the temperature sensing behavior of the synthesized phosphor has been studied by using FIR of two thermally linked levels of the central UC emission band against 980 nm excitation.
Er3+/Ho3+/Yb3+ UC phosphors via solution combustion method. Control of crystallization in the phosphors was studied by X-ray diffraction (XRD). The UC emission characteristics of the synthesized materials have been studied upon 980 nm diode laser excitation at room temperature. The effect of doping and the mechanism involved in the UC process has been studied in detail. The color of the infrared emissions of the codoped sample was also tuned by varying the laser power. Meanwhile, the temperature sensing behavior of the synthesized phosphor has been studied by using FIR of two thermally linked levels of the central UC emission band against 980 nm excitation.
Experimental section
Synthesis of La2CaZnO5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) Er3+, Ho3+, Yb3+ UC phosphors
Er3+, Ho3+, Yb3+ UC phosphors
The La2CaZnO5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+, Ho3+, Yb3+ UC phosphors (2 mol% Er3+, 2.0–20 mol% Yb3+, 1 mol% Ho3+, respectively) were synthesized via solution combustion route. Analytical reagent (AR) grade lanthanum acetate (C6H9LaO6, 99.9%), calcium nitrate (Ca(NO3)2, >99%), zinc nitrate (Zn(NO3)2, 97%), erbium nitrate (Er(NO3)3, 99.9%), holmium nitrate (Ho(NO3)3, 99.9%), ytterbium nitrate (Yb(NO3)3, 99.9%) and urea (CO(NH2)2, >90%) obtained from Sigma-Aldrich were used as the starting raw materials. These materials were taken in the required stoichiometric ratios and mixed properly with deionized water under vigorous stirring condition. When the transparent solution was obtained, the proportionate amount of urea was added to the solution and the solution was stirred for 20 min. Then the solution was transferred in an alumina crucible and kept in a preheated furnace (500 °C) for about 10 min. Within this time, the auto combustion process took place, and a very porous voluminous mass of the powder was formed. The resultant powder was then calcined at 800 °C for 1 h.
Er3+, Ho3+, Yb3+ UC phosphors (2 mol% Er3+, 2.0–20 mol% Yb3+, 1 mol% Ho3+, respectively) were synthesized via solution combustion route. Analytical reagent (AR) grade lanthanum acetate (C6H9LaO6, 99.9%), calcium nitrate (Ca(NO3)2, >99%), zinc nitrate (Zn(NO3)2, 97%), erbium nitrate (Er(NO3)3, 99.9%), holmium nitrate (Ho(NO3)3, 99.9%), ytterbium nitrate (Yb(NO3)3, 99.9%) and urea (CO(NH2)2, >90%) obtained from Sigma-Aldrich were used as the starting raw materials. These materials were taken in the required stoichiometric ratios and mixed properly with deionized water under vigorous stirring condition. When the transparent solution was obtained, the proportionate amount of urea was added to the solution and the solution was stirred for 20 min. Then the solution was transferred in an alumina crucible and kept in a preheated furnace (500 °C) for about 10 min. Within this time, the auto combustion process took place, and a very porous voluminous mass of the powder was formed. The resultant powder was then calcined at 800 °C for 1 h.
Characterizations
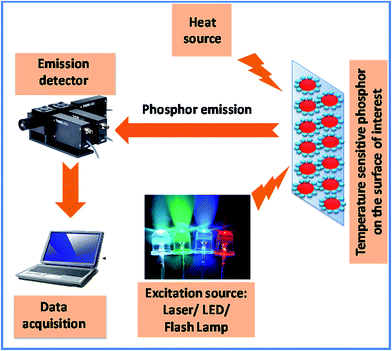
X-ray diffraction (XRD) patterns of the as-prepared phosphors were recorded using a Bruker D8 Focus X-ray diffractometer. The scanning electron microscope (SEM) analysis was carried out using a Shimadzu SSX-550. The UC emission and lifetime measurements were performed using the home built setup with a 980 nm laser source. The emitted light was dispersed into a monochromator coupled to a photomultiplier tube through the appropriate lens system. The sample was heated in a home-made furnace and the temperature was controlled by varying the voltage. The temperature was measured with the help of a calibrated thermocouple located in contact with the sample for the temperature sensing investigation. The sample holder along with the sample was kept for 5 min at a constant temperature unless its temperature was stabilized for the upconversion. A common thermometry unit based on phosphors has been illustrated in Scheme 1 schematically. An excitation source (such as LEDs, lasers or flash lamps) is used for the excitation of the phosphor which is attached onto the surface of interest. The resultant emission light passes through an optical filter and is collected in a data acquisition system via an emission detector.Results and discussion
Structures of the prepared samples
To examine the structure of the LCZ phosphor, the effect of Er3+-doping, Er3+/Yb3+ and Er3+/Yb3+/Ho3+-codoping in the crystal lattice and to identify the phase, XRD patterns of the UC phosphors were recorded and are shown in Fig. 1. The XRD peak matches well with the LCZ phase as reported elsewhere.16 The XRD pattern varying dopant and codopant show the same behavior indicating the invariance of the crystal structure after doping and codoping. The crystal system of the prepared samples was identified as a tetragonal structure with space group P4/mmm (no. 123) having lattice parameters a = b = 7.0837 Å, c = 11.884 Å and α = β = γ = 90°. It is evident from the existing literature that the iso-structures such as BaLa2ZnO5 and BaNd2ZnO5 derived via the sol–gel method follow the same tetragonal behavior.13a,14a,16Structure formation and unit cell
Rietveld refinement of long scan XRD patterns of undoped La2CaZnO5 was carried out and a part is shown here in Fig. 2(a) in the range ∼20–80°. The refinement was carried out by FullProf software using the XRD data. The experimental diffraction data is shown by black line. The “×” marks represent the simulated diffraction data. The blue line indicates how much the calculated data deviates from the original values. If this line will be straight it will be perfect matching. In the present case small noise in the blue line indicates good matching for this refinement. The eminence of refinement can be quantified by some factors obtained during refinement known as reliability factors. Reliability factor includes factor for the weighted pattern (Rwp), factor for the pattern (Rp), and the goodness-of-fit indicator (s). For good refinement, Rp and Rwp must be less than 10% and s must approach to unity. In the present case, the parameters were found to be well within the range of a good fitting as summarized in Table 1. The estimated reliability factors verified that the pure phases were obtained without impurities. The crystal system of the prepared samples was identified as a tetragonal structure with space group P4/mmm (no. 123) having lattice parameters a = b = 7.0837 Å, c = 11.884 Å and α = β = γ = 90°. The structural parameters obtained from the Rietveld analysis of the La2CaZnO5 phases are listed in Table 1. The refined structural parameters show good agreement with the previous work.13a,h,14a,16 The structure of the undoped LCZ was estimated via the VESTA software using the data obtained from the Rietveld refinement as shown in Fig. 2(b). The crystal structure of La2CaZnO5 is composed of three polyhedral LaO8, CaO10 and ZnO4 and the composition of three different polyhedra is schematically shown in Fig. 2(b).| Element | x | y | z | Frac. occup. |
|---|---|---|---|---|
| La (8p) | 0.17363 | 0.67363 | 0.0 | 1.00 |
| Zn (4i) | 0.0 | 0.5 | 0.25 | 1.00 |
| Ca (2g) | 0.0 | 0.0 | 0.25 | 1.00 |
| O(1) (1a) | 0.0 | 0.0 | 0.0 | 1.00 |
| O(2) (16u) | 0.3519 | 0.8519 | 0.1376 | 1.00 |
| a (Å) | 7.0837 | |||
| c (Å) | 11.8848 | |||
| Rwp, Rp, χ2 | 7.36%, 5.89% and 3.92 | |||
| Space group | P4/mmm (tetragonal) |
UC emission study
The UC of the as-synthesized LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Yb3+ phosphors were recorded as a function of different Yb3+-codoping concentrations and the corresponding results are shown in Fig. 3.
Er3+/Yb3+ phosphors were recorded as a function of different Yb3+-codoping concentrations and the corresponding results are shown in Fig. 3.
As shown in Fig. 3, three sensitized UC emission bands centered at 523, 548 and 672 nm were observed in the case of 2 mol% Er3+ doped LCZ phosphor. These bands corresponded to the transitions of Er3+ ions: 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2, respectively.16,21,22 With the increase in Yb3+ concentration the UC intensity was seen to increase and at the concentration of 2 mol% Er3+ and 5 mol% of Yb3+ the UC intensity reached a maximum. When the Yb3+ concentration was incorporated and increased, the green and red emission of Er3+ increased significantly, suggesting the appreciable energy transfer process between sensitizers (Yb3+) to the activators (Er3+). The Yb3+ ion has a pretty long absorption energy level (2F5/2 → 2F7/2) at 980 nm excitation wavelength. Therefore, upon excitation with 980 nm, apart from excited state absorption (ESA), an adequate energy transfer (ET) from Yb3+ to Er3+ could be conceivable due to spectral overlap between the Yb3+ transition of 2F5/2 → 2F7/2 and that of Er3+ absorption energy 4I11/2 → 4I15/2, which significantly increased the UC intensity. This is clearly shown in the energy-level diagram explained later. The digital image of the green colour emission is shown in the inset of Fig. 3.
Fig. 4 shows the UC spectra of LCZ: 2 mol% Er3+/5 mol% Yb3+, LCZ: 1 mol% Ho3+/5 mol% Yb3+ and LCZ: 2 mol% Er3+/1 mol% Ho3+/5 mol% Yb3+ phosphors. It is obvious that the green emission was more intense in the triply doped sample than that of other samples, which indicates efficient energy transfer between Yb3+, Er3+ and Ho3+ and the energy transfer mechanisms will be discussed later. The enhancement of UC emission after codoping with Ho3+ has been reported in other papers.23,24 To confirm the energy transfer phenomena the lifetime of the green emission of the LCZ samples with codoping ratio Er![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ho
Ho![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb as 0
Yb as 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 0
0, 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 2
5, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 2
0, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 2
5 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were recorded and are shown in Fig. 5. The resulting decay curves were found to follow the double exponential nature as per the following equation:25a
5 were recorded and are shown in Fig. 5. The resulting decay curves were found to follow the double exponential nature as per the following equation:25a
| I(t) = I1e−t/τ1 + I2e−t/τ2 | (1) |
 | ||
| Fig. 5 Decay intensity as function of time of the green emission (548 nm) of the LCZ samples with different codoping ratios. | ||
In this research, it is proven that Ho3+/Yb3+/Er3+ and Er3+/Yb3+ codoping samples are both suitable for UC emission by pumping at 980 nm, but the triply doped sample was even better. The close vicinity of the individual energy levels of these three ions make the energy transfer processes possible. To understand the mechanism precisely the UC intensity was recorded of LCZ: 2 mol% Er3+/5 mol% Yb3+ phosphor by varying the laser power (P). The intensity was seen to increase with the increase in P as shown in Fig. 6(a). Fig. 6(b) displays the similar increment behavior of the UC intensity of the LCZ: 2 mol% Er3+/1 mol% Ho3+/5 mol% Yb3+ phosphor while varying the laser power (P).
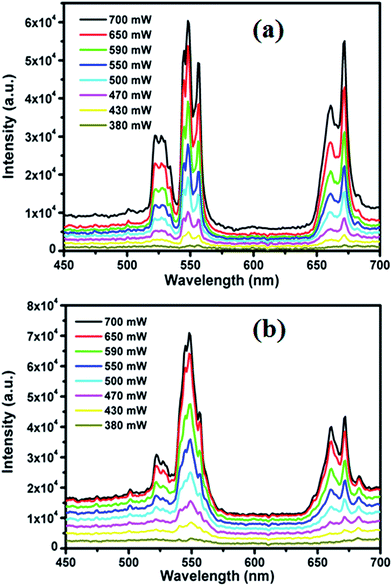 | ||
Fig. 6 Variation of UC intensity with the increment of the laser power for (a) LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb and (b) LCZ Er/Yb and (b) LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb phosphors. Er/Ho/Yb phosphors. | ||
The variation colour coordinate of LCZ: 2 mol% Er3+/5 mol% Yb3+ phosphor with P is shown in the Commission Internationale de L'Eclairage (CIE) chromaticity diagram of Fig. 7(a). The similar variation of colour coordinate from the yellowish green region towards the deep green region with the increase in pump power was also observed in the case of the LCZ: 2 mol% Er3+/1 mol% Ho3+/5 mol% Yb3+ phosphor as is shown in Fig. 7(b). It is well accepted that the temperature has been changed by varying the excitation power density, which in turn affect the emission intensities and colors. In this present research, the above possibility has been examined for LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb and LCZ
Er/Yb and LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb phosphors by measuring their emission colour and corresponding CIE coordinates as a function of different pump-power densities, as shown in Fig. 7. It shows that pump-power/temperature has a significant effect on the chromaticity coordinates, as a variation of colour coordinate from the yellowish green region towards the deep green region with the increase in pump power was observed in the case of the LCZ: 2 mol% Er3+/5 mol% Yb3+ and LCZ: 2 mol% Er3+/1 mol% Ho3+/5 mol% Yb3+ phosphors as shown in Fig. 7(a) and (b), respectively. Such variation in the CIE or emission colour may be accounted for due to the different saturation behavior of the green and red emissions of Er3+ and Ho3+ ions.25b The colour changing property of the present UC phosphors with the variation of power/temperature directly indicated their suitability for thermometry applications in which the emission of a phosphor need to be modulated very precisely under temperature variations.
Er/Ho/Yb phosphors by measuring their emission colour and corresponding CIE coordinates as a function of different pump-power densities, as shown in Fig. 7. It shows that pump-power/temperature has a significant effect on the chromaticity coordinates, as a variation of colour coordinate from the yellowish green region towards the deep green region with the increase in pump power was observed in the case of the LCZ: 2 mol% Er3+/5 mol% Yb3+ and LCZ: 2 mol% Er3+/1 mol% Ho3+/5 mol% Yb3+ phosphors as shown in Fig. 7(a) and (b), respectively. Such variation in the CIE or emission colour may be accounted for due to the different saturation behavior of the green and red emissions of Er3+ and Ho3+ ions.25b The colour changing property of the present UC phosphors with the variation of power/temperature directly indicated their suitability for thermometry applications in which the emission of a phosphor need to be modulated very precisely under temperature variations.
 | ||
Fig. 7 Variation of colour coordinates with the increment of laser power for (a) LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb and (b) LCZ Er/Yb and (b) LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb phosphors. Er/Ho/Yb phosphors. | ||
The intensity of the UC bands (Iemission) follows the relation: Iemission ∝ Pn, where n denotes the number of incident photons involved in the UC emission.16,21,22 The n value can be determined from the slope of the linear fitting between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I vs. log
I vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P as shown in Fig. 8. The straight line plot for three different bands and the slope (n) for LCZ: 2 mol% Er3+/5 mol% Yb3+ phosphors are found as 1.79 and 2.02 for the green transitions at 522, and 548 nm, and 2.48 for the red transition at 672 nm as shown in Fig. 8(a). In the case of LCZ: 2 mol% Er3+/1 mol% Ho3+/5 mol% Yb3+ phosphor the n value was obtained as 1.51 and 2.05 for the green transitions at 522, and 548 nm, and 1.99 for the red transition at 672 nm as shown in Fig. 8(b). The n values are much larger than 2 and revealed that two and three IR photons are involved in producing one green (548 nm) or red (672 nm) photon in this UC process, and the n values are close to 2 in the case of the 522 nm band, which indicates the involvement of two-photons as clearly explained in the literature.16
P as shown in Fig. 8. The straight line plot for three different bands and the slope (n) for LCZ: 2 mol% Er3+/5 mol% Yb3+ phosphors are found as 1.79 and 2.02 for the green transitions at 522, and 548 nm, and 2.48 for the red transition at 672 nm as shown in Fig. 8(a). In the case of LCZ: 2 mol% Er3+/1 mol% Ho3+/5 mol% Yb3+ phosphor the n value was obtained as 1.51 and 2.05 for the green transitions at 522, and 548 nm, and 1.99 for the red transition at 672 nm as shown in Fig. 8(b). The n values are much larger than 2 and revealed that two and three IR photons are involved in producing one green (548 nm) or red (672 nm) photon in this UC process, and the n values are close to 2 in the case of the 522 nm band, which indicates the involvement of two-photons as clearly explained in the literature.16
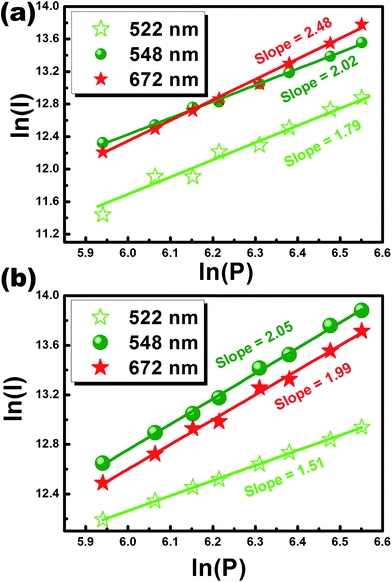 | ||
Fig. 8 Dependence of UC intensity with pumping power for (a) LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb and (b) LCZ Er/Yb and (b) LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb phosphors. Er/Ho/Yb phosphors. | ||
The energy level diagram showing the probable mechanism and population processes in LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Ho3+/Yb3+ UC phosphor is schematically shown in Fig. 9.16,21,22 The details of the different energy transfer processes in the present system were reported recently.16 The involved possible energy transfer mechanisms from Yb3+ and Er3+ to Ho3+ for the UC emission are discussed below based on the energy level diagram showed in Fig. 9. First, the 2F7/2 level of Yb3+ is excited to the 2F5/2 level by ground state absorption (GSA) when the sample is pumped by 980 nm laser and then can transfer their energy to the Ho3+
Er3+/Ho3+/Yb3+ UC phosphor is schematically shown in Fig. 9.16,21,22 The details of the different energy transfer processes in the present system were reported recently.16 The involved possible energy transfer mechanisms from Yb3+ and Er3+ to Ho3+ for the UC emission are discussed below based on the energy level diagram showed in Fig. 9. First, the 2F7/2 level of Yb3+ is excited to the 2F5/2 level by ground state absorption (GSA) when the sample is pumped by 980 nm laser and then can transfer their energy to the Ho3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5I6 and Er3+
5I6 and Er3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4I11/2 levels. GSA can also occur for Er3+
4I11/2 levels. GSA can also occur for Er3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4I15/2 → 4I11/2 when pumped by 980 nm laser. However, compared with the ground state absorption of Ho3+/Er3+, Yb3+ ions possess a larger absorption cross-section at 980 nm and ion concentration, and energy transfer occurs efficiently as a result of the large spectral overlap between the Yb3+ emission 2F5/2–2F7/2 and the Er3+ absorption 4I15/2–4I11/2 (ET1) or Ho3+ absorption 5I8–5I6 (ET2) bands. Because the energy gap (1040 cm−1) of the ET2 process is larger than that (432 cm−1) of the ET1 process, the energy transfer efficiency of ET1 is expected to be higher than that of ET2. Thus, ET1 can promote the Er3+ ion from the 4I15/2–4I11/2 state, and if the latter is previously populated, the Er3+ ion may transit from the 4I11/2 to the 4F7/2 and from 4F7/2 to 4G11/2 states. Succeeding nonradiative relaxations could fill the 2H11/2 and 4S3/2 states that are the emitting levels for the green luminescence.
4I15/2 → 4I11/2 when pumped by 980 nm laser. However, compared with the ground state absorption of Ho3+/Er3+, Yb3+ ions possess a larger absorption cross-section at 980 nm and ion concentration, and energy transfer occurs efficiently as a result of the large spectral overlap between the Yb3+ emission 2F5/2–2F7/2 and the Er3+ absorption 4I15/2–4I11/2 (ET1) or Ho3+ absorption 5I8–5I6 (ET2) bands. Because the energy gap (1040 cm−1) of the ET2 process is larger than that (432 cm−1) of the ET1 process, the energy transfer efficiency of ET1 is expected to be higher than that of ET2. Thus, ET1 can promote the Er3+ ion from the 4I15/2–4I11/2 state, and if the latter is previously populated, the Er3+ ion may transit from the 4I11/2 to the 4F7/2 and from 4F7/2 to 4G11/2 states. Succeeding nonradiative relaxations could fill the 2H11/2 and 4S3/2 states that are the emitting levels for the green luminescence.
 | ||
| Fig. 9 Energy level diagram of Er3+, Ho3+ and Yb3+ ions for various emissions of the La2CaZnO5 phosphor. | ||
The 4I11/2 states of Er3+ ions could be eliminated by an alternative relaxation process to 4I13/2 states, which may be further stimulated to the red emitting levels 4F9/2, and the identical transition to the ground state 4I15/2 gives red emission. In case of the Ho3+ ion, ET2 populated the 5I6 state of the Ho3+ ion which further promote the Ho3+ ion from the 5I6–5F4 state and the 5F4 state is the emitting levels for the green luminescence. If the 5I6 state of the Ho3+ ion depopulated by a nonradiative relaxation route to the 5I7 state, it can be excited to the 5F5 state of the Ho3+ ion which is the red emitting level.
But due to the stronger UC emission of Ho3+/Er3+/Yb3+ triply doped sample compared to other codoped samples, it can be inferred that there may exist some energy transfer process between the Er3+ and Ho3+ ions. The spectral overlap between the Er3+ emission 4S3/2–4I15/2 and the Ho3+ absorption 5F4–5I8 supports this conclusion. When Ho3+ ion is codoped in the LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb phosphor, the density of the Er3+ ions increases in the 4S3/2 level and hence the green (∼548 nm) emission increases.16,23,24 The possible energy migrations between Ho3+ and Er3+ can be verified via a comparative UC emission spectra of LCZ
Er/Yb phosphor, the density of the Er3+ ions increases in the 4S3/2 level and hence the green (∼548 nm) emission increases.16,23,24 The possible energy migrations between Ho3+ and Er3+ can be verified via a comparative UC emission spectra of LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb, LCZ
Er/Yb, LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ho/Yb and LCZ
Ho/Yb and LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb, as shown in Fig. 4. Substantial increase in the green-red intensity (I548 nm/I672 nm) ratio from 1.78 to 2.36 with the incorporation of Ho3+ in LCZ
Er/Ho/Yb, as shown in Fig. 4. Substantial increase in the green-red intensity (I548 nm/I672 nm) ratio from 1.78 to 2.36 with the incorporation of Ho3+ in LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb directly indicates the possible energy transfer between Er3+ and Ho3+. Now, the energy transfer process from Ho3+ to Er3+ in LCZ
Er/Yb directly indicates the possible energy transfer between Er3+ and Ho3+. Now, the energy transfer process from Ho3+ to Er3+ in LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb phosphor can be clearly confirmed from the lifetime analysis. The decay time for the 548 nm green emission were estimated to be 109, 113 and 137 μs for LCZ
Er/Ho/Yb phosphor can be clearly confirmed from the lifetime analysis. The decay time for the 548 nm green emission were estimated to be 109, 113 and 137 μs for LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb, LCZ
Er/Yb, LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ho/Yb and LCZ
Ho/Yb and LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb, respectively. As illustrated Fig. 5, an increment in the decay time for LCZ
Er/Ho/Yb, respectively. As illustrated Fig. 5, an increment in the decay time for LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb could be due to the energy transfer process from Ho3+ to Er3+. Nevertheless, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions (548 and 672 nm, respectively) of Er3+ ions are significantly overlapped with that of 5S2, 5F4 → 5I8 and 5F5 → 5I8 transitions (546 and 661 nm, respectively) of Ho3+ ions, as illustrated in Fig. 9. Owing to such overlapping, probabilities of energy transfer from Ho3+ and Er3+ enhanced. Therefore, it is predicted that such energy transfer phenomena may enhanced the green emission intensity and its life time in LCZ
Er/Ho/Yb could be due to the energy transfer process from Ho3+ to Er3+. Nevertheless, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions (548 and 672 nm, respectively) of Er3+ ions are significantly overlapped with that of 5S2, 5F4 → 5I8 and 5F5 → 5I8 transitions (546 and 661 nm, respectively) of Ho3+ ions, as illustrated in Fig. 9. Owing to such overlapping, probabilities of energy transfer from Ho3+ and Er3+ enhanced. Therefore, it is predicted that such energy transfer phenomena may enhanced the green emission intensity and its life time in LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb.
Er/Ho/Yb.
Temperature sensing ability
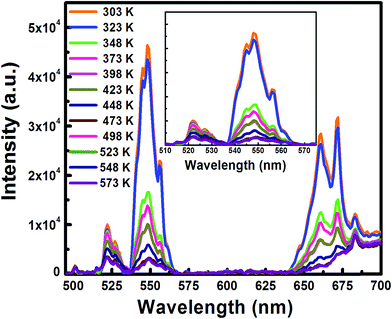
The temperature sensing study of UC emission spectra of the green bands from 300 K to 573 K of Er3+/Yb3+ codoped and Er3+/Ho3+/Yb3+ codoped (triply doped) LCZ phosphor have been performed with 980 nm excitation and are shown in Fig. 10 and 11, respectively. From both the figures, it is observed that on increasing the sample temperature, the band positions remain unchanged, but the integrated intensity of two green bands changed in a reverse way. At room temperature (300 K), the intensity corresponding to the 2H11/2 → 4I15/2 transition was lower than that of the 4S3/2 → 4I15/2 transition. While at a higher temperature (573 K), the reverse effect was observed.This variation in the intensity of the two bands that increased their intensity ratio (I522 nm/I548 nm) as a function of temperature was caused due to the change in their relative population.16 The ratio is called the Fluorescent Intensity Ratio (FIR) and the variation of FIR with temperature for both the sample is shown in Fig. 12.
 | ||
| Fig. 12 Variation of FIR value of the green emission in LCZ host with different doping as a function with temperature. | ||
As, the population of these two thermally coupled levels follows the Boltzmann's distribution, the FIR of these transitions can be used for optical thermometry via the relation:
FIR = I522/I548 = C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/kT) exp(−ΔE/kT)
| (2) |
The linear conversion of eqn (2) can be written as
| ln(I522/I548) = −(ΔE/k)(1/T) + ln(C) | (3) |
The corresponding energy difference ΔE can be calculated from the slope (ΔE/k) of the linear fitting of ln(I525 nm/I548 nm) versus 1/T plot as shown in Fig. 13. The fitting of the experimental data gives a slope of about 640.53 and 1168.85 for codoped and triply doped phosphors respectively and resulting in an energy difference ΔE of about 444 and 810 cm−1.16 The performance of the temperature sensor generally depends on the figure of merit of the sensing behavior. The figure of merit includes different parameters such as absolute sensitivity (Sa), relative sensitivity (Sr) and the resolution. The absolute sensitivity is defined as the variation of the FIR or lifetime (for two approaches) with respect to temperature and is expressed as:26
| S = dR/dT = FIR(−ΔE/kT2) | (4) |
 | ||
| Fig. 13 Variation of logarithmic FIR value of the green emission in the LCZ host with inverse temperature. | ||
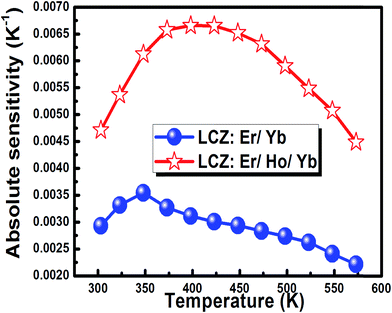
The absolute sensor sensitivity in the present case was calculated for the FIR measurements and was obtained as 0.0029 and 0.0047 K−1 at 300 K for the codoped and the triply doped phosphors, respectively and then increased with the rise in temperature. The maximum sensitivity was observed as 0.0036 at 348 K for the codoped phosphor whereas 0.0067 at 398 K for the triply doped phosphor. The sensitivity value was higher in the case of the triply doped sample. This is because after codoping of Ho3+, the Boltzmann population of the Er3+ ion increased in the 4S3/2 state. Therefore, the intensity ratio between the transition from 2H11/2 and 4S3/2 state to ground state as well as the FIR were modified, which affected the sensitivity.27 As illustrated in the energy diagram (Fig. 9), UC initiates through Yb3+ sensitizer excitation and ET to Er3+ and Ho3+ ions and consequently favors the simultaneous inter-ion ET between Er3+ and Ho3+ ions. Moreover, excited state absorption (ESA) and cross-relaxation (CR) processes in both Er3+ and Ho3+ ions significantly improve the UC efficiency. Now, as we know that the temperature sensitivity is depended on the FIR which is defined as the intensity ratio of the emission intensities centered at 525 nm and 548 nm. Due to the incorporation of Ho in the Er/Yb codoped system, this ratio has been changed and it is found that the FIR values of Er/Yb/Ho codoped system changed more systematically owing to which the sensitivity was increased.
With the further increase in temperature the sensitivity decreased for both the cases. The variation of absolute sensitivity with temperature for FIR measurement is shown in Fig. 14. This figure shows that the sensitivity increased after codoping of Ho3+ in the LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Yb3+ phosphor. The relative sensor sensitivity is the normalized absolute sensor sensitivity with respect to the measured value. The eqn (5) is used to calculate the relative sensitivity as per the following equation:28,29
Er3+/Yb3+ phosphor. The relative sensor sensitivity is the normalized absolute sensor sensitivity with respect to the measured value. The eqn (5) is used to calculate the relative sensitivity as per the following equation:28,29
 | (5) |
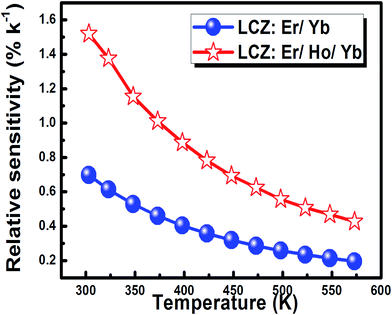
Fig. 15 indicates that the relative sensor sensitivity values decreased with temperature in the measured range. However, the values are sensibly higher than the reported over the examined large temperature range from room temperature to the higher temperatures. This behavior indicates the suitability of this material as an attractive host in the operation of electronic devices for temperature sensing purposes. The maximum relative sensor sensitivity value is 0.71% K−1 and 1.52% K−1 at 300 K for the codoped and the triply doped phosphors respectively.
The effect of Ho3+ concentration on the experimentally estimated sensitivity was determined for the 300–573 K temperature range and the variation of the maximum relative sensitivity at 300 K with Ho3+ concentration is presented in Fig. 16. The figure shows that with an increase of the Ho3+ ions amount from 1 to 9 mol% the relative sensitivity decreased exponentially from 1.52% K−1 to 0.28% K−1. This effect can be explained via the energy transfer phenomena between Ho3+ ions. With the increment of Ho3+ concentration, the cross relaxation [5F4,5I8] → [5I5,5I7] between Ho3+ ions starts which leads to lowering of the 2H11/2 state population of Er3+ and hence 2H11/2 → 4I15/2 emission intensity. The probability of this energy transfer increases with the reduction of the average distance between these ions connected. Therefore, the cross relaxation process will affect the changes of FIR and hence the relative sensitivity. Marciniak et al.30 has observed the similar behaviour in the case of Er![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiYbP4O12 luminescent thermometer.
LiYbP4O12 luminescent thermometer.
A comparison of relative sensor sensitivity value and the temperature range between the recently developed Ln3+ phosphor based inorganic nano-thermometers is summarized in Table 2 and it indicates that the relative sensor sensitivity value in case of the present phosphor is among the highest.
| S. No. | Phosphor | Sr at Tm | ΔT (Tm) | Ref. |
|---|---|---|---|---|
| 1 | UC Nps![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Yb3+ Er3+/Yb3+ |
2.3 | 293–318 (318) | 31 |
| 2 | UC Nps![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tm3+/Yb3+ Tm3+/Yb3+ |
0.2 | 293–318 (315) | 31 |
| 3 | NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Yb3+ Er3+/Yb3+ |
1.0 | 298–318 (298) | 20 |
| 4 | Gd2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Yb3+ Er3+/Yb3+ |
0.2 | 295–1000 (600) | 32 |
| 5 | Fluoride glass![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Yb3+ Er3+/Yb3+ |
1.1 | 333–375 (342) | 33 |
| 6 | ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Yb3+ Er3+/Yb3+ |
0.6 | 273–473 (273) | 34 |
| 7 | LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb Er/Yb |
∼0.51 (s = 0.0059 K−1) | 298–513 (483) | 16 |
| 8 | LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb Er/Yb |
0.71 (s = 0.0036 K−1) | 300–573 (300) | Present work |
| 9 | LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Yb/Ho Er/Yb/Ho |
1.52 (s = 0.0067 K−1) | 300–573 (300) | Present work |
Temperature resolution is also an important factor to characterize any temperature sensor devices and can be defined to the minimal detectable signal change. The standard deviation data of residuals in the fit of the experimental FIR data points with temperature and the absolute sensitivity were used to estimate resolution as the method described by Brites et al.18 and the estimated resolutions of FIR temperature sensing is shown in Fig. 17.
Both the curve follows similar behavior and it is observed that the FIR measurements for the codoped phosphors provided better resolution compared to the triply doped sample. The resolution values in both the cases are very much lower than 1 K over a large temperature range from 300 K to 573 K. The maximal resolutions were obtained at 325 K for the codoped phosphor as 0.105 K and at 425 K for the triply doped sample 0.115 K. The comparative sensitivity data and resolution behavior of the LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er/Ho/Yb phosphor with the other phosphor indicates the suitability of this material for temperature sensing application. Similar studies have been reported by Li and co-workers for sol–gel derived Yb3+–Er3+ co-doped La2CaZnO5 phosphors, with a sensitivity of 0.0059 K−1 at 483 K.16 But the FIR at 483 K is about 1.15. This indicates the Sr value is about 0.51% K−1. Similar studies were also carried out by various authors for different materials.9,17 In the present case for the sample LCZ : Er/Yb the sensitivity was observed as 0.0036 K−1 at 348 K. This value has increased by above 69% after codoping of Ho3+, which is about 7% greater than the reported one. The obtained relative sensitivity is higher than the reported. And also, the resolution is very much lower than 1 K over a large temperature range from 300 K to 573 K. The results imply that the studied UC phosphor is superb for temperature sensing applications.
Er/Ho/Yb phosphor with the other phosphor indicates the suitability of this material for temperature sensing application. Similar studies have been reported by Li and co-workers for sol–gel derived Yb3+–Er3+ co-doped La2CaZnO5 phosphors, with a sensitivity of 0.0059 K−1 at 483 K.16 But the FIR at 483 K is about 1.15. This indicates the Sr value is about 0.51% K−1. Similar studies were also carried out by various authors for different materials.9,17 In the present case for the sample LCZ : Er/Yb the sensitivity was observed as 0.0036 K−1 at 348 K. This value has increased by above 69% after codoping of Ho3+, which is about 7% greater than the reported one. The obtained relative sensitivity is higher than the reported. And also, the resolution is very much lower than 1 K over a large temperature range from 300 K to 573 K. The results imply that the studied UC phosphor is superb for temperature sensing applications.
Conclusions
In summary, a series of Er3+/Yb3+ and Er3+/Ho3+/Yb3+ codoped La2CaZnO5 phosphors were prepared by the combustion synthesis method. The crystal system of the prepared samples was identified as a tetragonal structure and the doping of RE ions did not affect the crystal structure. Under the laser excitation of 980 nm, the Er3+/Yb3+-codoped sample exhibited strong green and red UC emissions from 2H11/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 transitions of Er3+ ions, respectively. The enhanced UC emission after codoping of Ho3+ was explained on the basis of energy exchange mechanisms between Er3+/Ho3+ and Yb3+ ions. Power dependence studies infer that the UC bands arose through a two/three-photon absorption process. The high corresponding sensor responsiveness over a broad temperature range with large temperature resolution make the phosphors suitable for future applications in thermometry. The FIR technique was used to measure the sensitivity of the synthesized temperature sensors, and it can be concluded that at 400 K the obtained phosphors shows a relatively higher sensitivity and resolution over the existing one. These data indicate the suitability of LCZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er3+/Ho3+/Yb3+ phosphors for temperature sensor applications.
Er3+/Ho3+/Yb3+ phosphors for temperature sensor applications.
Acknowledgements
Dr Vijay Kumar is greatly acknowledged the Science and Engineering Research Board (SERB), New Delhi for financial assistance in the form of Young Scientist Scheme (Fast Track; File No. DST No. SB/FTP/ETA-215/2014). The financial assistance from the University of the Free State is also highly recognized.References
- H.-Q. Wang, M. Batentschuk, A. Osvet, L. Pinna and C. J. Brabec, Adv. Mater., 2011, 23, 2675–2680 CrossRef CAS PubMed.
- J. C. Ruiz-Morales, J. Mendez-Ramos, P. Acosta-Mora, M. E. Borgesc and P. Esparza, J. Mater. Chem. C, 2014, 2, 2944–2948 RSC.
- R. Martin-Rodríguez, R. Valiente, S. Polizzi, M. Bettinelli, A. Speghini and F. Piccinelli, J. Phys. Chem. C, 2009, 113(28), 12195–12200 Search PubMed.
- Y. Tian, B. Tian, C. Cui, P. Huang, L. Wang and B. Chen, RSC Adv., 2015, 5, 14123–14128 RSC.
- (a) W. Xu, Z. G. Zhang and W. W. Cao, Opt. Lett., 2012, 37, 4865–4867 CrossRef CAS PubMed; (b) R. Dey, A. Kumari, A. K. Soni and V. K. Rai, Sens. Actuators, B, 2015, 210, 581 CrossRef CAS; (c) A. K. Soni, V. K. Rai and S. Kumar, Sens. Actuators, B, 2016, 229, 476 CrossRef; (d) A. K. Soni and V. K. Rai, Dalton Trans., 2014, 43, 13563 RSC.
- (a) G. Ajithkumar, B. Yoo, D. E. Goral, P. J. Hornsby, A.-L. Lin, U. Ladiwala, V. P. Dravide and D. K. Sardar, J. Mater. Chem. B, 2013, 1, 1561–1572 RSC; (b) S. P. Tiwari, K. Kumar and V. K. Rai, J. Appl. Phys., 2015, 118, 183109 CrossRef.
- J. Chen and J. X. Zhao, Sensors, 2012, 12, 2414–2435 CrossRef CAS PubMed.
- B. Dong, B. Cao, Y. He, Z. Liu, Z. Li and Z. Feng, Adv. Mater., 2012, 24, 1987–1993 CrossRef CAS PubMed.
- B. P. Singh, A. K. Parchur, R. S. Ningthoujam, P. V. Ramakrishna, S. Singh, P. Singh, S. B. Rai and R. Maalej, Phys. Chem. Chem. Phys., 2014, 16, 22665–22676 RSC.
- S. Zhou, K. Deng, X. Wei, G. Jiang, C. Duan, Y. Chen and M. Yin, Opt. Commun., 2013, 91, 138–142 CrossRef.
- H. Zheng, B. Chen, H. Yu, J. Zhang, J. Sun, X. Li, M. Sun, B. Tian, H. Zhong, S. Fu, R. Hua and H. Xia, RSC Adv., 2014, 4, 47556 RSC.
- (a) X. Yang, Z. Fu, Y. Yang, C. Zhang, Z. Wu and T. Sheng, J. Am. Ceram. Soc., 2015, 98, 2595 CrossRef CAS; (b) V. K. Rai, Appl. Phys. B, 2007, 88, 297 CrossRef CASA. K. Soni, R. Dey and V. K. Rai, RSC Adv., 2015, 5, 34999 RSC; (c) A. Kumar, S. P. Tiwari, K. Kumar and V. K. Rai, Spectrochim. Acta, Part A, 2016, 167, 134 CrossRef CAS PubMed.
- (a) I. Etchart, M. Berard, M. Laroche, A. Huignard, I. Hernandez, W. P. Gillin, R. J. Curry and A. K. Cheetham, Chem. Commun., 2011, 47, 6263–6265 RSC; (b) B. N. Tian, B. J. Chen, Y. Tian, J. S. Sun, X. P. Li, J. S. Zhang, H. Y. Zhong, L. H. Cheng, Z. L. Wu and R. N. Hua, Ceram. Int., 2012, 38, 3537–3540 CrossRef CAS; (c) A. Jaffres, B. Viana and E. van der Kolk, Chem. Phys. Lett., 2012, 527, 42–46 CrossRef CAS; (d) I. Etchart, I. Hernandez, A. Huignard, M. Berard, M. Laroche, W. P. Gillin, R. J. Curry and A. K. Cheetham, J. Appl. Phys., 2011, 109, 063104 CrossRef; (e) C.-H. Liang, Y.-C. Chang and Y.-S. Chang, J. Electrochem. Soc., 2009, 156, J303 CrossRef CAS; (f) L. Shi and H. J. Seo, J. Lumin., 2011, 131, 523–525 CrossRef CAS; (g) M. J. J. Lammers, H. Donker and G. Blasse, Mater. Chem. Phys., 1985, 13, 527–529 CrossRef CAS; (h) J. Xie, L. Mei, L. Liao, M. Guan and H. Liu, J. Phys. Chem. Solids, 2015, 83, 152–156 CrossRef CAS; (i) A. Yoshida, H. Ogawa, A. Kan and T. Kondo, J. Eur. Ceram. Soc., 2005, 25, 2897–2900 CrossRef CAS.
- (a) V. R. Bandi, B. K. Grandhe, K. Jang, H.-S. Lee, D.-S. Shin, S.-S. Yi and J.-H. Jeong, J. Alloys Compd., 2012, 512, 264–269 CrossRef CAS; (b) H. J. Woo, V. R. Bandi, B. K. Grandhe, K. Jang, J. Park, J. Yoon, H. S. Lee, D. H. Bae, S. S. Yi and J. H. Jeong, J. Nanosci. Nanotechnol., 2013, 13(2), 848–852 CrossRef CAS PubMed.
- A. Birkel, A. A. Mikhailovsky and A. K. Cheetham, Chem. Phys. Lett., 2009, 477, 325–329 CrossRef CAS.
- L. Li, C. Guo, S. Jiang, D. K. Agrawal and T. Li, RSC Adv., 2014, 4, 6391 RSC.
- (a) A. Pandey, S. Som, V. Kumar, V. Kumar, K. Kumar, V. K. Rai and H. C. Swart, Sens. Actuators, B, 2014, 202, 1305 CrossRef CAS; (b) H. Suo, C. Guo, Z. Yang, S. Zhou, C. Duan and M. Yin, J. Mater. Chem. C, 2015, 3, 7379 RSC; (c) S. K. Singh, K. Kumar and S. B. Rai, Appl. Phys. B, 2010, 100, 443 CrossRef CAS.
- C. D. S. Brites, P. P. Lima, N. J. O. Silva, A. Millan, V. S. Amaral, F. Palacio and L. D. Carlos, Nanoscale, 2012, 4, 4799 RSC.
- D. Jaque and F. Vetrone, Nanoscale, 2012, 4, 4301 RSC.
- F. Vetrone, R. Naccache, A. Zamarron, A. J. de la Fuente, F. Sanz-Rodrıguez, L. M. Maestro, E. M. Rodriguez, D. Jaque, J. G. Sole and J. A. Capobianco, ACS Nano, 2014, 4, 3254–3258 CrossRef PubMed.
- W. Xu, Z. G. Zhang and W. W. Cao, Opt. Lett., 2012, 37, 4865–4867 CrossRef CAS PubMed.
- J. S. Wang, D. P. Machewirch, F. Wu, E. M. Vogel and E. Snitzer, Opt. Lett., 1994, 19, 1448–1449 CrossRef CAS PubMed.
- R. Xu, J. Pan, L. Hu and J. Jhang, J. Appl. Phys., 2010, 108, 043522–043527 CrossRef.
- Y. Tian, I. Hang, S. Feng, R. Xu, L. Hu and J. Jhang, Opt. Mater., 2010, 32, 1508–1513 CrossRef CAS.
- (a) S. Dutta, S. Som and S. K. Sharma, RSC Adv., 2015, 5, 7380 RSC; (b) S. Das, A. Amarnath Reddy, S. Surendra Babu and G. Vijaya Prakash, Mater. Lett., 2014, 120, 232 CrossRef CAS.
- S. A. Wade, S. F. Collins and G. W. Baxter, J. Appl. Phys., 2003, 94, 4743 CrossRef CAS.
- H. Zheng, B. Chen, H. Yu, J. Zhang, J. Sun, X. Li, M. Sun, B. Tian, H. Zhong, S. Fu, R. Hua and H. Xia, RSC Adv., 2014, 4, 47556–47563 RSC.
- C. H. Hsia, A. Wuttig and H. Yang, ACS Nano, 2011, 5, 9511–9522 CrossRef CAS PubMed.
- E. J. McLaurin, V. A. Vlaskin and D. R. Gamelin, J. Am. Chem. Soc., 2011, 133, 14978–14980 CrossRef CAS PubMed.
- L. Marciniak, K. Waszniewska, A. Bednarkiewicz, D. Hreniak and W. Strek, J. Phys. Chem. C, 2016, 120(16), 8877–8882 CAS.
- N. N. Dong, M. Pedroni, F. Piccinelli, G. Conti, A. Sbarbati, J. E. Ramirez-Hernandez, L. M. Maestro, M. C. Iglesias-de la Cruz, F. Sanz-Rodriguez, A. Juarranz, F. Chen, F. Vetrone, J. A. Capobianco, J. G. Sole, M. Bettinelli, D. Jaque and A. Speghini, ACS Nano, 2011, 5, 8665–8671 CrossRef CAS PubMed.
- S. K. Singh, K. Kumar and S. B. Rai, Sens. Actuators, A, 2009, 149, 16–20 CrossRef CAS.
- E. Saidi, B. Samson, L. Aigouy, S. Volz, P. Low, C. Bergaud and M. Mortier, Nanotechnology, 2009, 20, 115703 CrossRef PubMed.
- X. Wang, X. G. Kong, Y. Yu, Y. J. Sun and H. Zhang, J. Phys. Chem. C, 2007, 111, 15119–15124 CAS.
| This journal is © The Royal Society of Chemistry 2016 |