Biomimetic fabrication of biotinylated peptide nanostructures upon diatom scaffold; a plausible model for sustainable energy†
Vikas Kumar‡
a,
Shradhey Gupta‡ a,
Avin Rathodb,
Vandana Vinayak*b and
Khashti Ballabh Joshi*a
a,
Avin Rathodb,
Vandana Vinayak*b and
Khashti Ballabh Joshi*a
aDepartment of Chemistry, Dr. Harisingh Gour Central University Sagar (MP), 470003, India. E-mail: kbjoshi77@gmail.com; kbjoshi@dhsgsu.ac.in
bDepartment of Forensic Science and Criminology, Dr. Harisingh Gour Central University Sagar (MP), 470003, India
First published on 25th July 2016
Abstract
This study demonstrates the interactions of a biotinylated peptide with single cell eukaryotic microalgae known as diatoms, present in most natural water resources. The specifically designed biotinylated peptide consists of two key components, tryptophan along with the biotin, which helps us to unravel the interactions of this peptide with diatoms via spectroscopic tools owing to the high affinity biotin–avidin interactions and unique photophysical and photochemical properties of the Trp residue. The spectroscopic observations are further confirmed by atomic force microscopy (AFM), where the interactions of the biotinylated peptide afford a unique nanoarray over the diatom frustules. This peptide soft structure has potential for the encapsulation of gold nanoparticles which can be disrupted upon exposure of sunlight/radiation; hence this peptide can act as an adequate metal nanoparticle carrier for the diatom. Owing to thermoplasmonic heat of nanoparticles, the peptide can stimulate diatom frustules to open up, allowing oil to be extracted. Thus we hypothesize a most simple and parsimonious model for alternative/renewable energy resources and light-harvesting assemblies.
The importance of bionanotechnology is nowadays burgeoning in the field of modern research. Small scale biological operating systems are emerging business these days, as bio-nanotechnology becomes the backbone of most sectors – through the use of biosensors, biopharmaceuticals, and biomaterials.1–5 The design, synthesis and manipulation of biomolecules is rapidly gaining importance in a number of areas, such as biomedical sciences, drug and gene delivery, energy science, optoelectronics, and catalysis,6–8 to name a few. Biomolecular recognition, specific interactions between two or more molecules, can be approached from different perspectives and is a cornerstone for the advancement of bionanotechnolgy.9–11 Bioactive small molecules such as peptides, either natural products or synthetic compounds, can be useful probes to identify the desired target for relevant interactions. Researchers often show interests that lead to the development of methodology to identify biomolecular interactions and its application.12–14
Short peptides are a special class of bioactive compounds; they can be enriched with desired functionality and can show high interaction potential with biomolecules such as membrane proteins and thus with several types of cells, including bacterial and algal cells, with great biocompatibility.15–20 This special class of peptides contain several non-covalent interaction sites which interact with the target system when in close proximity. The conformational changes upon interaction with the target system can be determined easily, which is sometimes difficult with later ones.21,22 We recently designed a peptide composed of one unit each of D-biotin and di-L-tryptophan peptide; this biotinylated peptide, 1, is known to be rapidly self-assembled into vesicular structures23 which show potential application in drug delivery vehicles (Fig. 1). These vesicular structures were further used for in situ synthesis and encapsulation of gold nanoparticles followed by thermoplasmonic disruption of these peptide vesicles.24 The design of such a peptide is attributed to high-affinity biotin–avidin interaction, where the contributions of three Trp residues control the exceptionally high binding constant of biotin–avidin complex formation.25,26 This peptide can be used to deliver gold nanoparticles into cells and hence can work as a theranostic agent too.
The interaction module between human erythrocytes and biotin had already established;27,28 therefore this time we wished to look at the interaction of this peptide with microalgal cells, i.e. diatoms. An alternative to diatoms may be mesoporous silica, which, although it has excellent drug delivery quality, is limited as a drug delivery vehicle for toxic chemicals, is time-consuming and economically not cost-effective. Therefore, comparatively, diatoms have proven to be an excellent natural alternative with similar potential.29
Diatoms are unicellular photosynthetic algae that range in size from 5 μm to 5 mm, found in almost all open water bodies and divided into two main groups, centric and pinnate. Centric have radial and pinnate have bilateral symmetry.29 In both types of diatoms there are two almost equal-sized walls known as the hypotheca and epitheca which fit onto each other like the lid onto a Petri dish. The walls are embedded with silica girdle bands which form the nanoporous silica shells.30–33 These silica valves are like glass plates which trap solar energy just like any other optical fibre. Diatom silica is composed of amorphous silica and several organic compounds e.g. long chain polyamines, silafins, silacidins, cingulins, frustulins and polysaccharides.34 Diatoms alone among algae are considered as the primary producers in phytoplanktons, responsible for 25% CO2 fixation,35 and are major biofuel producers, producing 30% crude oil. Oil in diatoms is rich in polyunsaturated fatty acids and a major source of renewable energy, but owing to the thick silica wall the extraction of oil is cumbersome and requires high energy input for its production. Here we propose the interaction of 1 with a pure culture of diatom cells Nitzschia palea grown in f/2 culture medium36,37 as a possible model for renewable energy.38–41
Recently we demonstrated that compound 1 exhibits significant solution-phase self-assembly to afford extensive vesicular structures, mainly aided by the interaction of the biotin and aromatic Trp residues. In this context, intramolecular hydrogen-bonding of biotin in solution, with the participation of the aromatic π interactions, was described. The diatom cell wall is also made up of several proteins, e.g. frustulins, silaffins, silacidins, lipids and polyamines, which are perhaps potential target binding sites for peptide(s). With this background, we decided to study the interactions of diatoms with 1 in solution. We started this work by first culturing the diatoms in modified f/2 medium36 followed by fixing of freshly isolated diatoms on glass slides. The fixed diatoms were imaged in dry conditions and the size and shape were first studied by optical microscopy (OM) and scanning electron microscopy (SEM) (Fig. 2) followed by atomic force microscopy (AFM) (Fig. 3A and B). The SEM micrographs clearly reveal neat and clean diatoms of various sizes and shapes possessing a wonderful nanoarchitecture with a homogeneous ordered arrangement of their open pores. The high resolution AFM micrograph further confirmed the observations obtained from SEM and corresponded well with the SEM analysis. Diatoms represent an exciting source of potential applications in pharmaceuticals, in cosmetics utilizing intracellularly synthesized fatty acids and amino acids, and as power-harvesting devices.
 | ||
| Fig. 3 (A) High resolution atomic force microscopic image of open pores, and (B) corresponding 3D micrograph depicting homogeneous ordered arrangement of pores of the diatoms. | ||
The unique and intricate frustule of the diatom was used to test the resolution of optical microscopes.42,43 Diatoms are cultivated in large quantities for use as food for marine organisms such as shrimps,44 and therefore researchers from material science and nanotechnology fields have great interest in diatom frustules owing to their symmetrical and hierarchical pore structure. The frustules of diatoms can therefore be proposed to provide a template for 3D-engineered nanostructured materials (Fig. 3).
Our recent advances in research motivated us to check the potential application of this short peptide, 1. Peptide 1 shows strong interaction with metal nanoparticles and therefore used for encapsulation of AuNPs.24 Based on our great interest in bionanotechnology23,24,45 this time we wished to explore the interaction of 1 with diatom cells by recording the UV-Vis spectrum (Fig. 4). The preliminary investigations clearly indicate that the non-covalent interaction sites, i.e. protein, of the diatom cell wall are responsible for the binding of this small molecule (Fig. 4). The diatom cell wall is made up of a peptide–silica envelope and therefore contains several non-covalent interaction sites, such as aromatic side chains, charged amino acids and other important functional groups. These interaction sites are perhaps useful and can show strong interactions with small molecules such as biotinylated peptides, such as 1. In order to confirm any role of non-covalent molecular interactions between the tryptophan-containing short peptide and diatoms, we performed a fluorescence study (Fig. 1). Most tryptophan-containing peptides and proteins exhibit an intrinsic fluorescent nature unique among other biological molecules that can be used for biomolecular recognition, and have great advantage in bionanotechnology. Here, short peptide 1 was used as a fluorescent probe to provide evidence of non-covalent interactions by fluorescence titration. We experimentally observed the change in fluorescence intensity upon the gradual addition of diatoms to a solution of 1 (see Fig. 1, ESI†). The fluorescence intensity was quenched many-fold without affecting the shape of the emission spectrum. It is our belief that the observed quenching of fluorescence intensity is a result of a photo-induced electron transfer (PET) process between the peptide molecule and cell wall proteins of diatoms, which clearly reveals that 1 interacts with diatom cells and the probable site of interaction is the Trp–Trp residue (ESI†). To obtain the fluorescence quenching quantitatively, the quenching constant was determined by the Stern–Volmer equation (ESI†). A representative Stern–Volmer plot in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (50
EtOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) binary solvent mixture is shown in Fig. 5a. The observed value of the Stern–Volmer constant was found to be 9.14 × 10−10 diatom−1. To calculate the quenching rate constant of bimolecular interactions, we measured the lifetime of the tryptophan-based short peptide molecule fluorescence, shown in Fig. 5b. The decay profile of the tryptophan-based short peptide molecule was well fitted by a bi-exponential function and the experimental average lifetime obtained was approximately 2.68 ns. Hence, the bimolecular quenching rate constant (kq) was determined as 3.21 × 10−1 s−1 diatom−1. This quantitative study of fluorescence quenching furnished solid evidence of the existence of non-covalent interactions (Fig. 5 and 1). This observation corresponds well with binding between tryptophan-containing peptide and protein.
50) binary solvent mixture is shown in Fig. 5a. The observed value of the Stern–Volmer constant was found to be 9.14 × 10−10 diatom−1. To calculate the quenching rate constant of bimolecular interactions, we measured the lifetime of the tryptophan-based short peptide molecule fluorescence, shown in Fig. 5b. The decay profile of the tryptophan-based short peptide molecule was well fitted by a bi-exponential function and the experimental average lifetime obtained was approximately 2.68 ns. Hence, the bimolecular quenching rate constant (kq) was determined as 3.21 × 10−1 s−1 diatom−1. This quantitative study of fluorescence quenching furnished solid evidence of the existence of non-covalent interactions (Fig. 5 and 1). This observation corresponds well with binding between tryptophan-containing peptide and protein.
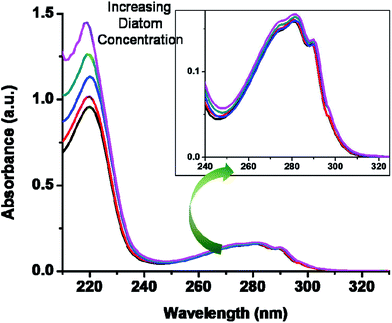 | ||
| Fig. 4 UV-Vis titration spectra of compound 1 in the presence of increasing concentrations of diatom (N. palea) solution depict the interaction of 1 with the diatom cell wall. The concentration of diatoms was ∼40 × 106 diatom cells/10 μL solution.48 | ||
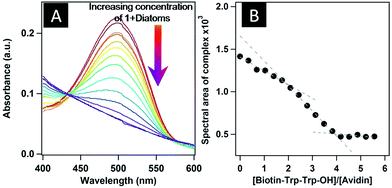
HABA, 2-[(4-hydroxyphenyle)azo]benzoic acid, a dye that differs structurally from biotin, also binds to avidin46 with a much lower affinity (Ka = 104 M−1), and hence is displaced from the binding pocket of its avidin complex when treated with biotin or biotinylated conjugates. Therefore, with this background, we also wished to check the binding of preformed vesicles of 1 with diatoms by HABA assay. The absorption maximum of HABA, which is at 348 nm, was red-shifted to 500 nm upon binding with avidin (Fig. 6). When HABA-saturated avidin was treated with 1, the absorption maximum decreased and the HABA was replaced by 1, and interestingly we also found 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 complex formation (Fig. 6B) between avidin and 1 for which the binding constant was observed to be 6.13 × 1018 M−4 (ESI†). The back-titration of a HABA-saturated solution of avidin (HA-complex) with peptide-bound diatoms displayed a decrease in the UV-Vis absorbance at 500 nm as a result of HABA being expelled from the binding sites in avidin (Fig. 7A, ESI†). This observation suggests that 1 binds to surface protein of diatoms and by using the diatom surface as a template generates hybrid structures. Interestingly, two inflection points were obtained during the back-titration, appearing at N1 = ∼1.85 and N2 = ∼3.93. The Job's plot (Fig. 7B) clearly indicates that there is a stepwise binding mechanism between 1 and avidin in the presence of diatoms; however, no such observation was obtained in back-titration without diatoms (ESI†). To the best of our knowledge and literature survey, we have observed for the first time the stepwise binding of our biotinylated peptide with avidin in the presence of diatoms. In general a 1
4 complex formation (Fig. 6B) between avidin and 1 for which the binding constant was observed to be 6.13 × 1018 M−4 (ESI†). The back-titration of a HABA-saturated solution of avidin (HA-complex) with peptide-bound diatoms displayed a decrease in the UV-Vis absorbance at 500 nm as a result of HABA being expelled from the binding sites in avidin (Fig. 7A, ESI†). This observation suggests that 1 binds to surface protein of diatoms and by using the diatom surface as a template generates hybrid structures. Interestingly, two inflection points were obtained during the back-titration, appearing at N1 = ∼1.85 and N2 = ∼3.93. The Job's plot (Fig. 7B) clearly indicates that there is a stepwise binding mechanism between 1 and avidin in the presence of diatoms; however, no such observation was obtained in back-titration without diatoms (ESI†). To the best of our knowledge and literature survey, we have observed for the first time the stepwise binding of our biotinylated peptide with avidin in the presence of diatoms. In general a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 avidin–biotin complex has been known; however, our experimental results depict formation of a 1
4 avidin–biotin complex has been known; however, our experimental results depict formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with avidin followed by formation of a 1
2 complex with avidin followed by formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 complex, when treated with HABA-saturated avidin. This suggests that, at first, two HABA molecules are expelled from the binding pocket of avidin, certainly mediated/guided by diatoms. After the formation of the 1
4 complex, when treated with HABA-saturated avidin. This suggests that, at first, two HABA molecules are expelled from the binding pocket of avidin, certainly mediated/guided by diatoms. After the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex, subsequently at higher concentration of peptide-bound diatom sample, the remaining two HABA molecules are expelled from the avidin pocket, finally leading to the formation of the 1
2 complex, subsequently at higher concentration of peptide-bound diatom sample, the remaining two HABA molecules are expelled from the avidin pocket, finally leading to the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 complex. The stepwise binding mechanism was consistent for the overnight incubated sample of peptide and diatoms, further supporting this observation (ESI†).
4 complex. The stepwise binding mechanism was consistent for the overnight incubated sample of peptide and diatoms, further supporting this observation (ESI†).
 | ||
| Fig. 7 (A) Back-titration of HABA-saturated avidin with co-incubated sample of 1 with diatoms in water, and (B) Job's plot showing the stepwise binding of 1 in the presence of diatom solution. | ||
Our spectroscopic observations demonstrated that molecules of compound 1 interact with diatoms and perhaps the favoured sites for interaction are pores. Therefore these diatoms can also provide the template for the symmetrical and hierarchical assembly of the soft structure of 1. To validate this hypothesis we have co-incubated the solution of diatoms and peptide at ambient temperature for 3–4 h followed by centrifugation. After processing the sample (ESI†), 5 μL aliquots were fixed onto a freshly cleaned glass surface by using methanol as fixing agent. The AFM images reveal that selective and precise deposition begins near and inside the pores of diatom frustules (Fig. 8). We assumed that this is the stage where the pre-organization of self-assembled structures is seen and is a nucleation phase for the next step in which the system attains maximum energy, and therefore not the stable and final saturated state. However, such selective deposition of self-assembled structures was clearly and primarily guided by the diatom cell surface, which provided a unique template for making the hierarchical nanoarray of peptide vesicles (Fig. 8). We should also like to mention here that the diatoms are stable under normal physiological conditions, which is a major advantage in using these cells for other potential applications.
The diatom surface is made up of a silicio-peptide envelope known as a frustule. This silicio-peptide envelope helps diatoms in the mineralization process and it can be used for in situ synthesis and deposition of metal nanoparticles over the surface of diatoms. It has already been mentioned that the cell membrane of diatoms contains various proteins together with embedded silica. The protein envelope, enriched with amino acids with specific functionality, is responsible for the interaction of diatoms with bioactive compounds. The siliceous envelope of diatoms supports the self-assembly process of various inorganic and organic molecules, and fascinating nanoarchitecture emerges. The preliminary spectroscopic and microscopic investigations of diatom and diatom–peptide samples motivated us to check the morphological changes on the diatom surface upon prolonged incubation. Interestingly, upon prolonged incubation (10–12 h) the nanoarray of peptide vesicles was clearly visible over the diatom surface. The sample(s) was loaded on the glass surface (ESI†) followed by imaging. The AFM images clearly demonstrate that diatoms are loaded with peptide vesicles (Fig. 9A and B). The high magnification AFM images showed the diatom frustules decorated with peptide vesicles (Fig. 9C and E) in an abacus-like assembly. This can be clearly seen in 3D micrographs of prolonged-incubation diatom–peptide hybrid structures (Fig. 9D and F). The high resolution AFM images give more insight into these beautiful nanostructures and show that the diatom surface was nicely decorated by the peptide nanostructures (Fig. 9).
To check the stability of biotinylated peptide nanostructures on the diatom surface, we performed one new experiment. A sample of peptide–diatoms co-incubated for more than 30 days at ambient temperature without further dilution and in the absence of other additives was used. Aliquots (10 μL) of this sample were transferred onto freshly cleaved mica surfaces and dried, followed by imaging by atomic force microscopy. Since mica is hydrophilic in nature, we successfully loaded the sample onto it without using any fixing agent. Further, using mica as a new substrate allowed us to check the stability of these hybrid structures in the presence of a hydrophilic substrate. Interestingly, our observations revealed that the nanostructures of 1 were stable over the diatom surface even after >30 days of incubation and the nanoarray of these nanostructures was intact and more refined (Fig. 10).
On the other hand, we also observed that in a few diatoms the deposition was in excess and therefore the nanostructures of 1 were deposited randomly. This observation reveals that even though there is random deposition of nanostructures over the diatom surface the biotinylated peptide nanostructures are stable under these physiological conditions and did not show any major deformations in either diatoms or soft nanostructures of 1 (ESI†).
Thus, we have developed a simple, efficient and practical method for the interaction of peptide with diatoms under physiological conditions. The self-assembled preformed structures of peptide 1 were decorated over the diatom surface and formed a beautiful nanoarray of peptide vesicles. The diatom surfaces provided a template for hierarchical nanoarray and served as a scaffold, and were investigated by various spectroscopic and microscopic tools. Such interactions and homogeneous deposition of peptide soft structure can be very useful for production of biofuels from diatoms and can work as a unique model for energy/light-harvesting assemblies.3 This peptide is also known for encapsulation of gold nanoparticles,24,46,47 which can be disrupted upon exposure to sunlight/radiation. Hence this peptide can act as a carrier to attach metal nanoparticles to diatoms. Owing to the thermoplasmonic heat of nanoparticles, pores of the diatom wall will open up so that oil oozes from it. Thus we hypothesize a most simple and parsimonious model for the production of renewable energy. Further, such special classes of hybrid structure are easy to handle owing to their stability and can therefore be used as diagnostic agents and treatments for various ailments too. These reported results could be of great interest and our continuous efforts in this direction will provide a new model for the production of biofuel as a source of sustainable energy.
Experimental section
General
Avidin and HABA were purchased from Sigma-Aldrich and used without further purification. Milli-Q water, ethanol, and other general chemicals were purchased from Spectrochem, Mumbai, India, and used without further purification.Peptide synthesis
Biotinylated peptide (1) used in this study was synthesized by using the standard protocol detailed in a research paper published by our group.1,2 Purity and identity were confirmed prior to use.Atomic force microscopy (AFM)
Untreated and co-incubated samples (at 37 °C for 0–1 day in ethanol/water) of diatoms and peptide–diatom solution, respectively, were imaged with an atomic force microscope. The solution was incubated at ambient temperature for 3–12 h followed by centrifuging. The supernatant, which contained the unbound peptide and diatoms, was decanted. The residue (pellet) was washed with methanol followed by Milli-Q water and redissolved in an appropriate amount of Milli-Q water. Aliquots (5 μL) of this sample were fixed onto freshly cleaned glass surfaces by using methanol as fixing agent. The samples were dried in a dust-free atmosphere under a 60 W lamp for 4 h followed by high-vacuum drying, and subsequently examined under an atomic force microscope (AFM) (INNOVA, ICON Analytical Equipment, Bruker, Sophisticated Instrument Center – Dr. Harisingh Gour Central University, Sagar-M.P.) operated in acoustic AC mode (AAC or tapping mode), with the aid of a cantilever (NSC 12(c) from MikroMasch, silicon nitride tip) by NanoDrive™ version 8 software. The force constant was 2.0 N m−1, while the resonance frequency was ∼280 kHz. The images were taken in air at room temperature, with a scan speed of 1.0–1.5 lines/sec. The data analysis was done using Nanoscope Analysis Software. The sample-coated substrates were dried in a dust-free atmosphere under a 60 W lamp for 6 h followed by high-vacuum drying, and subsequently examined under AFM.Scanning electron microscopy (SEM)
Aliquots (20 μL) of fresh and aged samples of diatoms (number of diatoms ∼4 × 106/μL) were dried at room temperature on a glass mirror (5 × 5 × 1 mm3) surface and coated with gold. Scanning electron microscopy images were made using an FEI QUANTA 200 microscope equipped with a tungsten filament gun operating at WD 10.6 mm and 20 kV.Fluorescence studies
Fluorescence spectra were recorded on an RF-5301 PC spectrofluorophotometer (Shimadzu) with a 10 mm quartz cell at room temperature. Fluorescence lifetime was measured by the single-photon counting method using a commercial setup from Jobin-Yvon. For lifetime measurement, samples were excited at 287 nm using a nano-LED.Uv-Vis experiments
UV-Vis absorption spectra were recorded on a LabIndia UV-VIS spectrophotometer 3000+ with 10 mm quartz cell at room temperature.Procedure for cell culture of diatoms and preparation of permanent slides
A water sample was collected from the Bebas River, Sagar district (PIN: 470003), M.P, India and after centrifugation at 4000 rpm for 5 min and further cleaning with Milli-Q water, the pellet was inoculated into modified f/2 medium.36 Diatom colonies obtained were serially diluted to obtain axenic cultures of Nitzschia palea. The number of diatom cells was counted using a Neubauer chamber Bürker, 1.00 mm depth, 1.00 mm2 on the 10th day of culture (ESI†).2Cell count
The diatom cells were counted using a Neubauer chamber.48Acknowledgements
VK thanks NFOBC-UGC-India for a pre-doctoral research fellowship. SG thanks CSIR-India for postdoctoral (RA) research assistance. AR and VV thank DBT India for financial assistance. The authors thank SIC-DHSGU Sagar-India and IITK for use of the AFM/SEM facility and lifetime measurements respectively. Our acknowledgment would be incomplete without thanking Prof. Sandeep Verma, IIT Kanpur for his constant encouragement and invaluable support. This work was supported by CSIR-India project no. 02(0238)/15/EMR-II to KBJ.Notes and references
- A. Gulzar, S. Gai, P. Yang, C. Li, M. B. Ansari and J. Lin, J. Mater. Chem. B, 2015, 3(44), 8599–8622 RSC.
- C. J. Bowerman and B. L. Nilsson, J. Am. Chem. Soc., 2010, 132(28), 9526–9527 CrossRef CAS PubMed.
- A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40(8), 644–656 CrossRef CAS PubMed.
- S. Si, R. R. Bhattacharjee, A. Banerjee and T. K. Mandal, Chem.–Eur. J., 2006, 12, 1256–1265 CrossRef CAS PubMed.
- P. R. Selvakannan, S. Mandal, S. Phadtare, A. Gole, R. Pasricha, S. D. Adyanthaya and M. Sastri, J. Colloid Interface Sci., 2004, 269, 97–102 CrossRef CAS PubMed.
- G. A. Hudalla, T. Sun, J. Z. Gasiorowski, H. Han, Y. F. Tian, A. S. Chong and J. H. Collier, Nat. Mater., 2014, 13(8), 829–836 CrossRef CAS PubMed.
- T. Sun, H. Han, G. A. Hudalla, Y. Wen, R. R. Pompano and J. H. Collier, Acta Biomater., 2016, 30, 62–71 CrossRef CAS PubMed.
- C. I. Cheng, Y. P. Chang and Y. H. Chu, Chem. Soc. Rev., 2012, 41, 1947–1971 RSC.
- S. R. Forrest, Chem. Rev., 2007, 107(4), 923–925 CrossRef CAS.
- C. T. Lin, M. T. Kao, K. Kurabayashi and E. Meyhofer, Nano Lett., 2008, 8(4), 1041–1046 CrossRef CAS PubMed.
- Z. Tang, J. P. P. Hernandez, W. C. Law, Z. E. Hughes, M. T. Swihart, P. N. Prasad, M. R. Knecht and T. R. Walsh, ACS Nano, 2013, 7(11), 9632–9646 CrossRef CAS PubMed.
- J. F. Acheson, L. J. Bailey, N. L. Elsen and B. G. Fox, Nat. Commun., 2014, 5(5009), 1–9 Search PubMed.
- D. D. Boehr, R. Nussinov and P. E. Wright, Nat. Chem. Biol., 2009, 5, 789–796 CrossRef CAS PubMed.
- E. Pazos, O. Vázquez, J. L. Mascareñas and M. E. Vázquez, Chem. Soc. Rev., 2009, 38, 3348–3359 RSC.
- K. A. Drouvalakis, S. Bangsaruntip, W. Hueber, L. G. Kozar, P. J. Utz and H. Dai, Biosens. Bioelectron., 2008, 23(10), 1413–1421 CrossRef CAS PubMed.
- S. Naahidi, Y. Wang, M. Zhang, R. Wang, M. Jafari, Y. Yuan, B. Dixon and P. Chen, Mol. Pharm., 2014, 11(10), 3409–3420 CrossRef CAS PubMed.
- A. Roy, O. L. Franco and S. M. Mandal, Curr. Protein Pept. Sci., 2013, 14(7), 580–587 CrossRef CAS PubMed.
- J. B. Matson and S. I. Stupp, Chem. Commun., 2011, 47, 7962–7964 RSC.
- M. Fani, H. R. Maecke and S. M. Okarvi, Theranostics, 2012, 2(5), 481–501 CrossRef CAS PubMed.
- L. Geng, Z. Wang, X. Yang, D. Li, W. Lian, Z. Xiang, W. Wang, X. Bu, W. Lai, Z. Hu and Q. Fang, Theranostics, 2015, 5(10), 1154–1165 CrossRef CAS PubMed.
- H. Chen, G. Niu, H. Wu and X. Chen, Theranostics, 2016, 6(1), 78–92 CrossRef CAS PubMed.
- L. D. Fricker, J. S. Gelman, L. M. Castro, F. C. Gozzo and E. S. Ferro, J. Proteome Res., 2012, 11(3), 1981–1990 CrossRef CAS PubMed.
- K. B. Joshi and S. Verma, Angew. Chem., Int.Ed., 2008, 47, 2860–2905 CrossRef CAS PubMed.
- N. K. Mishra, V. Kumar and K. B. Joshi, RSC Adv., 2015, 5, 64387–64394 RSC.
- D. Dong, D. Zheng, F. Q. Wang, X. Q. Yang, N. Wang, Y. G. Li, L. H. Guo and J. Cheng, Anal. Chem., 2004, 76(2), 499–501 CrossRef CAS PubMed.
- J. Collot, J. Gradinaru, N. Humbert, M. Skander, A. Zocchi and T. R. Ward, J. Am. Chem. Soc., 2003, 125(30), 9030–9031 CrossRef CAS PubMed.
- K. B. Joshi, V. Venkatesh and S. Verma, Chem. Commun., 2010, 46, 3890–3892 RSC.
- Y. Gottlieb, O. Topaz, L. A. Cohen, L. D. Yakov, T. Haber, A. Morgenstern, A. Weiss, K. C. Berman, E. Fibach and E. G. Meyron-Holtz, Haematologica, 2012, 97(7), 994–1002 CrossRef PubMed.
- V. Vinayak, V. Mishra and M. K. Goyal, J. Forensic. Res., 2013, 4(5), 1–6 Search PubMed.
- J. T Chao, M. J. P. Biggs and A. S. Pandit, Expert Opin. Drug Delivery, 2014, 11(11), 1687–1695 CrossRef PubMed.
- S. Chandrasekaran, M. J. Sweetman, K. Kant, W. Skinner, D. Losic, T. Nann and N. H. Voelcker, Chem. Commun., 2014, 50, 10441–10444 RSC.
- T. Todd, Z. Zhen, W. Tang, H. Chen, G. Wang, Y. J. Chuang, K. Deaton, Z. Pan and J. Xie, Nanoscale, 2014, 6, 2073–2076 RSC.
- I. E. Pamirsky and K. S. Golokhvast, Mar. Drugs, 2013, 11, 3155–3167 CrossRef PubMed.
- B. Delalat, V. C. Sheppard, S. R. Ghaemi, S. Rao, C. A. Prestidge, G. McPhee, M. L. Rogers, J. F. Donoghue, V. Pillay, T. G. Johns, N. Kröger and N. H. Voelcker, Nat. Commun., 2015, 6(8791), 1–11 Search PubMed.
- R. Gordon, D. Losic, M. A. Tiffany, S. S. Nagy and F. A. Sterrenburg, Trends Biotechnol., 2009, 27(2), 116–127 CrossRef CAS PubMed.
- V. Vinayak, R. Gordon, S. Gautam and A. Rai, Adv. Sci. Lett., 2014, 20(7–9), 1256–1267 CrossRef.
- R. R. L. Guillard and J. H. Ryther, J. Microbiol., 1962, 8, 229–239 CAS.
- Y. Maeda, T. Tateishi, Y. Niwa, M. Muto, T. Yoshino, D. Kisailus and T. Tanaka, Biotechnol. Biofuels, 2016, 9(10), 1–9 Search PubMed.
- J. M. Graham, L. E. Graham, S. B. Zulkifly, B. F. Pfleger, S. W. Hoover and J. Yoshitani, J. Ind. Microbiol. Biotechnol., 2012, 39(3), 419–428 CrossRef CAS PubMed.
- T. Mutanda, D. Ramesh, S. Karthikeyan, S. Kumari, A. Anandraj and F. Bux, Bioresour. Technol., 2011, 102(1), 57–70 CrossRef CAS PubMed.
- C. Jeffryes, J. Campbell, H. Li, J. Jiao and G. Rorrer, Energy Environ. Sci., 2011, 4, 3930–3941 CAS.
- D. Losic, K. Short, J. G. Mitchell, R. Lal and N. H. Voelcker, Langmuir, 2007, 23(9), 5014–5021 CrossRef CAS PubMed.
- Y. Yu, J. A. Mensah and D. Losic, Langmuir, 2010, 26(17), 14068–14072 CrossRef CAS PubMed.
- T. Lebeau and J. M. Robert, Appl. Microbiol. Biotechnol., 2003, 60(6), 612–623 CrossRef CAS PubMed.
- N. K. Mishra, V. Kumar and K. B. Joshi, Nanoscale, 2015, 7, 20238–20248 RSC.
- K. B. Joshi and S. Verma, Biophys. Chem., 2009, 140, 129–132 CrossRef CAS PubMed.
- N. K. Mishra, K. B. Joshi and S. Verma, J. Colloid Interface Sci., 2015, 455, 145–153 CrossRef CAS PubMed.
- I. Moreno-Garrido, M. Hampel, L. Lubián and J. Blasco, Fresenius' J. Anal. Chem., 2001, 371, 474–478 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13657e |
| ‡ Equal contribution for this work. |
| This journal is © The Royal Society of Chemistry 2016 |







