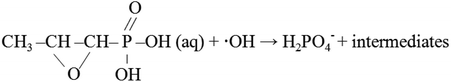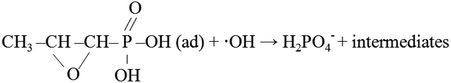Fosfomycin removal and phosphorus recovery in a schorl/H2O2 system†
Jing Shia,
Danyang Yinb,
Zhengwen Xu*b,
Duanmei Songa and
Feng Caoa
aSchool of Engineering, China Pharmaceutical University, Nanjing, 211198, P. R. China
bSchool of Environmental Science and Engineering, Nanjing University of Information Science and Technology, Nanjing 210044, P. R. China. E-mail: zwxu@nuist.edu.cn
First published on 14th July 2016
Abstract
A schorl/H2O2 system was developed for fosfomycin removal and phosphorus recovery. The phosphorus removal and recovery performances and mechanisms were investigated. The results indicated that more than 90% organic phosphorus could be removed. The organic phosphorus converted into inorganic phosphorus by the Fenton-like oxidation, and about one-third inorganic phosphorus could be adsorbed on the schorl. Moreover, inorganic phosphorus greatly competed with organic phosphorus for the adsorption sites on the schorl. Oxidation and adsorption presented a synergistic effect on the phosphorus removal and recovery. Heterogeneous catalytic oxidation played an important role during the oxidation process. The kinetic results showed that the BMG model was more suitable to describe the organic phosphorus removal in comparison with the pseudo-first-order and pseudo-second-order kinetic models. The results of zeta potential, infrared spectroscopy and X-ray photoelectron spectroscopy analyses indicated that the weak electrostatic interaction between the phosphate and the attracted H+ or H3O+ on the schorl was the main adsorption mechanism. In the schorl/H2O2 system, schorl was not only the supplier for the catalyst, but also the carrier for phosphorus recovery, which proposed a new idea to degrade harmful organic pollutants and simultaneously recover useful resources.
1. Introduction
Eutrophication has become a worldwide and serious problem due to the high levels of phosphorus plus nitrogen in rivers and lakes. The excessive discharged phosphorus in water bodies leads to algal blooms, resulting in the reduction of available oxygen and the deterioration of water quality.1 The traditional biological phosphorus removal system is considered as an economical and sustainable method for phosphorus removal from wastewater. However, it is not suitable for some organic phosphorus (OP) wastewater, such as pharmaceutical wastewater, because of the toxicity and low biodegradability.Fosfomycin (1R,2S-epoxypropyl phosphonic acid), which possesses the C–P in its chemical structure, is a broad spectrum antibiotic. It exhibits an effective antimicrobial activity against Gram positive and Gram negative bacteria.2 During its pharmaceutical process, the finished products, fosfomycin, is one of the main pollutants in the wastewater.3 Moreover, due to the incomplete metabolization of the antibiotic in human or animal body,4 a portion of the fosfomycin is excreted as original or metabolized forms into the environment. These could not only affect the aquatic organisms, but also take excessive phosphorus into the river and lake, which could lead to the eutrophication.5 As mentioned before, due to the low biodegradability, the biological phosphorus removal system could not treat fosfomycin directly and effectively. Furthermore, to our knowledge, only a few related researches focus on the disposal of fosfomycin wastewater,3,6 so the new and effective methods for fosfomycin removal is urgently in need.
On the other hand, phosphorus itself is an important non-renewable and indispensable resource.7 Consequently, if the fosfomycin could be converted into inorganic phosphorus (IP) and be recovered, it not only removes the organic phosphorus in the wastewater but also realizes the sustainable utilization of phosphorus.
Schorl/H2O2 system is a mineral-catalyzed Fenton-like system. The schorl is a kind of tourmaline, which is a complex borosilicate mineral compounded with many elements and could be written as XY3Z6[Si6O18][BO3]W4. Tourmaline has spontaneous surface electric fields and self-radiating far-infrared, and it can release negative ions.8,9 If the Y in the formula was occupied by Fe2+, it is called as the schorl.10 The schorl/H2O2 system has been investigated mainly for the degradations of dyes.11–14 In this experiment, the schorl/H2O2 system was performed to remove the fosfomycin and recover phosphorus at the same time. Due to the Fe in the schorl, it could supply the catalyst for the oxidation, which could make the fosfomycin convert into IP. In addition, because of the containing Fe and –OH, it was supposed that a part of the IP possibly could be adsorbed on the schorl, reaching the aim of phosphorus removal and recovery simultaneously. In other words, the schorl may play a dual role in the schorl/H2O2 system. It was not only the supplier for catalyst, but also the carrier for phosphate recovery. So, the objective of the study was: (1) to determine the feasibility of utilizing the schorl/H2O2 system to remove OP and recover phosphorus simultaneously; (2) to clarify the possible mechanism.
Accordingly, in this experiment, the performances in the only schorl, only H2O2 and schorl/H2O2 systems were compared. The effect of pH was investigated, and the characteristics of OP conversion were observed and analyzed. Moreover, the original and used schorl were examined for the analysis of the possible mechanism in the schorl/H2O2 system.
2. Materials and method
2.1 Materials
Fosfomycin sodium, hydrogen peroxide solution (30 wt% in H2O), sodium hydroxide, potassium dihydrogen phosphate and tert-butyl alcohol were utilized in this experiment. All chemicals are of analytical grade and were purchased from Nanjing Shengjianquan Chemical Co., Ltd (Nanjing, China) except for fosfomycin sodium, which was obtained from Sigma-Aldrich. For reference, the structure of fosfomycin sodium was shown in the ESI Fig. S1.† The schorl was from Xinjiang Province of China. Prior to use, the schorl was washed with distilled water for several times and then dried at 313 K for 48 h.2.2 Experimental procedure
The degradation experiments were carried out in a constant-temperature water-bathing shaker, which kept at 60 °C. 100 mL fosfomycin sodium solution (about 16 mg-P/L, TP0), which had been adjusted at an appropriate pH by 0.1 M HCl or NaOH solutions, was added into conical flask (250 mL). 1.0 g schorl and 0.1 mL hydrogen peroxide were both added for the reaction of the schorl/H2O2 system. As for the schorl only and H2O2 only systems, the schorl or hydrogen peroxide was introduced into the fosfomycin sodium solution separately. Samples were taken at various time intervals. Total phosphorus in the solution (TPsol), inorganic phosphorus in the solution (IPsol), and total organic carbon (TOC) were determined immediately after filtration with 0.22 μm filters.Desorption experiment was performed by adding 100 mL 5% (w/v) NaOH. Then the mixtures were stirred vigorously at room temperature for about 10 min. The samples were filtration with 0.22 μm filters, then were measured and calculated for the total IP contents (TIP). TP desorption rate was 96–104%.
2.3 Analytical methods
The original and used schorl were analyzed by scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD). IP was measured with the molybdate blue method, and the TP were determined using persulfate digestion molybdate–ascorbic acid colorimetric method. Fe2+ content was followed by the 1,10-phenantroline colorimetric method.15 TOC were performed on a Shimadzu TOC analyzer.2.4 Mass balance calculations
Organic phosphorus content in the solution (OPsol) was determined by subtracting the IPsol content from the TPsol.3 Inorganic phosphorus adsorbed on the schorl (IPads) content was calculated by subtracting the IPsol content from the TIP. In summary, the relationship between the phosphorus data was illustrated in ESI Fig. S2.† And the equations for calculating the OPsol, IPads and OPads were listed as follows according to mass balance.| OPsol = TPsol − IPsol | (1) |
| IPads = TIP − IPsol | (2) |
| OPads = TP0 −TIP − OPsol | (3) |
Phosphorus removal efficiencies were showed as eqn (4) and (5).
 | (4) |
 | (5) |
2.5 Kinetic models
To investigate the kinetic characteristics of the schorl/H2O2 process, the pseudo-first-order, pseudo-second-order and Behnajady–Modirshahla–Ghanbery (BMG) kinetic models were applied to fit the experimental data. The mathematical equations were listed as the follows.16–18Pseudo-first-order kinetic model:
 | (6) |
Pseudo-second-order kinetic model:
 | (7) |
BMG model:
 | (8) |
3. Results and discussion
3.1 Characterization of the schorl
The XRD spectrum of the sample was shown in ESI Fig. S3.† Comparing with the standard PDF database, it matched the card no. 43-1464, demonstrated that it is the typical pattern of schorl. The results of FTIR analysis (ESI Fig. S4†) were consistent with the published papers. The band at 3628 cm−1 and 3556 cm−1 can be assigned to O–H stretching.19 The morphology of the schorl was analyzed by SEM shown in ESI Fig. S5.† The schorl particles presented irregular shapes and the size was about 1–20 μm. Observed from ESI Fig. S5,† it was clear that Si, Al and Fe oxides were the main components of the schorl.3.2 Organic phosphorous (OP) removal performance comparisons
A set of experiments performed by adding H2O2, schorl and both of them to fosfomycin aqueous solutions at initial pH = 3. TPsol and IPsol were the major indicators for the treatment efficiencies (Fig. 1). As shown in Fig. 1, the OPsol removal efficiency was about 23.0% in the sole schorl system. Meanwhile, IPsol values were found to be ignorable for the lack of oxidant. In the H2O2 system, the negligible IPsol values indicated the transformation of OP to IP has hardly taken place, even though H2O2 is a strong oxidant. In contrast, in the schorl/H2O2 system, OPsol removal rate was improved significantly, which was more than 90%. Furthermore, the TOC removal rate was about 45%. The results clearly indicated that the oxidation reaction occurred, resulting in phosphorus conversion, which showed the superiority of the schorl/H2O2 combination over other treatment processes above.3.3 Effect of pH
The effect of pH on OPsol removal rates were showed in Fig. 2(a). Better removal performances were achieved at lower pH, which were similar with the conclusions obtained from some other Fenton-like systems.20–22 This experimental phenomenon could be possibly explained in three aspects. Firstly, at lower pH, the proton could promote dissolution of Fe from the oxide surface, in favor of the homogeneous Fenton oxidation of fosfomycin. Secondly, at lower pH, the decomposition of H2O2 could produce more effective oxidant species, whereas at higher pH the H2O2 self-decomposition could occur and generate more non-reaction oxidant species.23,24 Thirdly, the influence of pH on the electrostatic effects between the organic material and the charged iron oxide surfaces could affect the heterogeneous oxidation.25 | ||
| Fig. 2 Effect of pH: (a) OPsol removal rates at different pH values. (b) The percentages of adsorbed phosphorus at different pH values in the schorl and schorl/H2O2 systems. | ||
The percentages of adsorbed phosphorus under different pH values in the schorl/H2O2 system were depicted in Fig. 2(b). Obviously, the adsorption capacity decreased remarkably with the increase of pH value.
In general, this sorption behavior versus pH could be attributed to a combination of surface charge characteristics of the schorl and the pH-dependent speciation of sorbate.26,27 So, the zeta potential of the schorl was determined. As expected, with the increased pH, it became more negative, with a point of zero charge (PZC) of about pH 2.5.
When the pH was equal or greater than 5, the predominant species were H2PO4− and HPO42−. The electrostatic repulsion between fosfomycin or phosphate and negatively charged schorl surface reduced phosphorus adsorption. Decreasing pH values results in slightly negative charged, in the form of more H2PO4− and H3PO4.28 In addition, at lower pH, a part of the epoxy structure could open,2 which possibly benefit for the adsorption. Although both schorl and phosphorus were negative at higher pH, a small amount of phosphorus was still adsorbed. This indicated the electrostatic interaction was not the only reason of adsorption. From previous researches, phosphorus could be adsorbed on the metal oxide via the formation of monodentate or bidentate surface complexes.29–31 Because of the Fe and Al element in the schorl, the above mechanism may be one of the reasons for the adsorption. However, due to its low adsorption amount at high pH values, it was not the main mechanism.
3.4 Kinetic model analysis
Three kinetic model equations, the pseudo-first-order, pseudo-second-order and BMG, were utilized to investigate the fitting of experimental data from the shorl/H2O2 process. The obtained kinetic model parameters by linear regressions are given in Table 1. Apparently, all the R2 values for BMG model were higher than other models. Consequently, BMG kinetic model is suitable to describe the organic phosphorus removal at different pH values by the schorl/H2O2 process. This was in accordance with the previous published kinetic results about the Fenton system.16,18 The 1/m and 1/b values suggested the initial removal rates and the theoretical maximum oxidation capacity of the schorl/H2O2 process at the end of the reaction, respectively. Obviously, they reached the highest values when the pH was 3, which was in agreement with the experiment results under different pH values.| pH | Pseudo-first-order | Pseudo-second-order | BMG | ||||
|---|---|---|---|---|---|---|---|
| k1 (h−1) | R2 | k2 (L mg−1 h−1) | R2 | m (h) | b | R2 | |
| 3 | 0.241 | 0.859 | 0.052 | 0.969 | 1.725 | 0.947 | 0.999 |
| 5 | 0.033 | 0.936 | 0.002 | 0.973 | 17.77 | 1.930 | 0.974 |
| 7 | 0.024 | 0.846 | 0.001 | 0.887 | 20.33 | 2.811 | 0.964 |
| 9 | 0.017 | 0.635 | 0.001 | 0.691 | 20.7 | 5.024 | 0.995 |
| 11 | 0.007 | <0 | 0.000 | <0 | 15.02 | 14.98 | 0.998 |
3.5 Phosphorus removal and recovery mechanisms in the schorl/H2O2 system
The OPads values firstly increased then reduced, but the IPads kept increasing. The reasons were summarized as follows: (1) at the initial time, the adsorption rate of OP exceeded over the rates of OP degradation and desorption; (2) due to the oxidation reaction, the increase in IPads was correlated to an enhancement in IP sorption driving force at the solid–liquid interface. Whereas, because of the oxidation resulting in sharp decrease of OPsol value, the driving potential for OP absorption declined with time, therefore the adsorption of OP slowed down; (3) simultaneously, the adsorbed OP could be oxidized, converted into IP directly; (4) furthermore, IP greatly competed with OP for the adsorption site on the schorl (see Section 3.5.2).
Based on the analysis above, in the schorl/H2O2 system, the OP could be possibly removed in two pathways. One, OP was transformed into IP after the rupture of C–P bond by oxidation. The other, OP and IP could be adsorbed on the schorl. Adsorption and oxidation existed simultaneously. As both the pH and the leaching Fe ion concentration were low, the precipitation between Fe ion and phosphate were neglected.28,32
Because fosfomycin could not be oxidized directly by only H2O2 (Fig. 1), the hydroxyl radical, generated from the schorl-catalyzed Fenton-like reaction was responsible for the degradation of OP. To elucidate the function of hydroxyl radical in OP conversion, tert-butanol was added into the schorl/H2O2 system with the concentration of 50 mg L−1 as the hydroxyl radical scavenger.34,35 The OPsol removal efficiency largely decreased to 53.3% in the presence of tert-butanol. This result confirmed the important role of hydroxyl radical for the OP degradation.
Moreover, oxidation by the hydroxyl radical could generate from homogeneous and heterogeneous catalytic reactions. In order to clarify the contributions, the conventional Fenton experiment was performed. Schorl was replaced by the ferrous ion, with the concentration of 0.6 mg L−1, which was the maximum value monitored in the schorl/H2O2 system. In the homogeneous catalytic experiment, only 29.7% OP was converted into IP, whereas the value was 91.8% in the schorl/H2O2 system. It was clear that the heterogeneous catalytic oxidation reaction was the significant mechanism.
In order to find the functional group for the adsorption and oxidation, the FTIR and XPS spectra were utilized for analysis. By comparison, a small peak at ∼2950 cm−1 and a board band centered at ∼3040 cm−1 was observed after the reaction in the schorl/H2O2 system (Fig. 5), which could be assigned to the stretching –OH vibration (ν(O–H⋯O)) of the water when strongly perturbed by hydrogen bonding or the ν(H3O+).36–38 This could attribute to the negative charged surface of schorl, which was easy to attract the H+ or H3O+. Consequently, the H2PO4− or HPO42− could be adsorbed by the weak electrostatic interaction. Moreover, the O 1s XPS spectra of the schorl before and after reaction were analyzed, which could be deconvoluted into two peaks. They were located at 530.9 eV and 531.7 eV, probably to X–![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) (oxygen bonded to Fe, Al or Si) and X–
(oxygen bonded to Fe, Al or Si) and X–![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (hydroxyl bonded to Fe, Al or Si).39,40 As shown in Fig. 6(a), after reaction the bonding energies shifted upward to 531.2 eV and 532.0 eV, respectively. It was attributed to the formation of hydrogen bond that caused the electronic density in O decreased.41 The above results demonstrated the H+ or H3O+ could be attached to the surface of schorl. Therefore, phosphorus could be adsorbed by the electrostatic interaction. This was also in agreement with the better phosphorus removal performance at the lower pH, and was also in consistent with the phenomenon that pH value increased with time during the reaction.
(hydroxyl bonded to Fe, Al or Si).39,40 As shown in Fig. 6(a), after reaction the bonding energies shifted upward to 531.2 eV and 532.0 eV, respectively. It was attributed to the formation of hydrogen bond that caused the electronic density in O decreased.41 The above results demonstrated the H+ or H3O+ could be attached to the surface of schorl. Therefore, phosphorus could be adsorbed by the electrostatic interaction. This was also in agreement with the better phosphorus removal performance at the lower pH, and was also in consistent with the phenomenon that pH value increased with time during the reaction.
 | ||
| Fig. 5 FTIR spectra of the schorl before (blue line) and after (red line) reaction: (a) the peak at ∼2950 cm−1. (b) The peak at ∼3556 cm−1. (c) The peaks at 400–1400 cm−1. | ||
In addition, the peak at 3556 cm−1 was shifted 3563 cm−1 after reaction, indicating that the –OH group, located at the corners of brucite-like fragments of three edges-sharing octahedral, participated in the reaction. The change of the –OH peak was similar with the observations attained from other metal oxide system for phosphorus adsorption.42 The peak at ∼417 cm−1, assigned as the stretching O–Fe–O vibration at octahedral sites,43,44 was shifted to ∼419 cm−1. It could be connected with the oxidation and iron dissociation during the action. The peaks at ∼476 cm−1, ∼751 cm−1, ∼777 cm−1, ∼1027 cm−1 were also shifted a little, which could be in connection with the function groups such as Si–O, O–Si–O, Si–O–Si45–47 during the reaction.
After the reaction, the P2p peak obviously showed up at 133.6 eV (Fig. 6(b)), indicating that phosphorus was detected on the surface of the schorl, which was consistent with the variation of TPsol concentration. Based on the above results, a probable mechanism of the reaction in the schorl/H2O2 system is listed as follows,14,26,30 and the possible transformation pathways for the OP illustrated in ESI Fig. S6.†
Homogeneous Fenton:
| Fe2+ + H2O2 → Fe3+ + ˙OH + H+; |
| Fe3+ + H2O2 → Fe2+ + HO2˙ + H+; |
Heterogeneous Fenton:
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) + H2O2 → Fe(II) + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) + ˙OH + H+; Fe(III) + ˙OH + H+; |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(III) + H2O2 → Fe(III) + H2O2 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe(II) + HO2˙ + H+; Fe(II) + HO2˙ + H+; |
Oxidation:
Adsorption:
4. Conclusion
The schorl/H2O2 system was utilized for fosfomycin removal and phosphorus recovery. About 91.8% OP could be converted into IP, and 33.5% IP could be adsorbed on the schorl. IP greatly competed with OP for the adsorption site on the schorl, which was benefit for phosphorus recovery. The conversion of OP into IP was mainly due to the heterogeneous catalytic oxidation. Compared with the pseudo-first-order and pseudo-second-order kinetic models, the experimental data of the organic phosphorus removal are described well with BMG model. The results of zeta potential, FTIR and XPS analyses indicated that the weak electrostatic interaction between the phosphate and the attracted H+ or H3O+ on the schorl was the main adsorption mechanism. In conclusion, the schorl played a dual role in the schorl/H2O2 system. It was not only the supplier for catalyst, but also the carrier for phosphate recovery. This brought a new idea to degrade harmful organic pollutants and simultaneously recover useful resources.Acknowledgements
This research was supported by the National Natural Science Foundation of China (51408612), the Natural Science Foundation of Jiangsu Province (BK20140660), the Qing Lan project and the Fundamental Research Funds for the Central Universities (2015PT002).References
- M. Lürling and F. van Oosterhout, Water Res., 2013, 47, 6527–6537 CrossRef PubMed.
- K. Chruszcz-Lipska, K. K. Zborowski, E. Podstawka-Proniewicz, S. Liu, Y. Xu and L. M. Proniewicz, J. Mol. Struct., 2011, 986, 49–56 CrossRef CAS.
- G. Qiu, Y. Song, P. Zeng, S. Xiao and L. Duan, Chemosphere, 2011, 84, 241–246 CrossRef CAS PubMed.
- M. Xia, A. Li, Z. Zhu, Q. Zhou and W. Yang, J. Environ. Sci., 2013, 25, 1291–1299 CrossRef CAS.
- D. J. Conley, H. W. Paerl, R. W. Howarth, D. F. Boesch, S. P. Seitzinger, K. E. Havens, C. Lancelot and G. E. Likens, Science, 2009, 323, 1014–1015 CrossRef CAS PubMed.
- G. Wang, D. Wang and X. Xu, et al., J. Hazard. Mater., 2012, 217, 366–373 CrossRef PubMed.
- D. P. Van Vuuren, A. F. Bouwman and A. H. W. Beusen, Global Environ. Change, 2010, 20, 428–439 CrossRef.
- R. R. Yeredla and H. Xu, J. Phys. Chem., 2008, 112, 532–539 CAS.
- L. D. Tijing, A. Amarjargal, Z. Jiang, M. T. G. Ruelo, C. H. Park, H. R. Pant, D. W. Kim, D. H. Lee and C. S. Kim, Curr. Appl. Phys., 2013, 13, 205–210 CrossRef.
- P. S. R. Prasad, Gondwana Res., 2005, 8, 265–270 CrossRef CAS.
- H. Y. Xu, S. Y. Qi, Y. Li, Y. Zhao and J. W. Li, Environ. Sci. Pollut. Res., 2013, 20, 5764–5772 CrossRef CAS PubMed.
- H. Y. Xu, T. N. Shi, L. C. Wu and S. Y. Qi, Water, Air, Soil Pollut., 2013, 224, 1–11 CrossRef CAS.
- C. Wang, Y. Zhang, L. Yu, Z. Zhang and H. Sun, J. Hazard. Mater., 2013, 260, 851–859 CrossRef CAS PubMed.
- H. Xu, M. Prasad and Y. Liu, J. Hazard. Mater., 2009, 165, 1186–1192 CrossRef CAS PubMed.
- A. D. Eaton, L. S. Clesceri and A. E. Greenberg, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, DC, 22nd edn, 1995 Search PubMed.
- S. Tunc, T. Gürkana and O. Dumanb, Chem. Eng. J., 2012, 181–182, 431–442 CrossRef CAS.
- N. Bensalaha, A. Khodary and A. Abdel-Wahab, J. Hazard. Mater., 2011, 189, 479–485 CrossRef PubMed.
- M. A. Behnajady, N. Modirshahla and F. Ghanbary, J. Hazard. Mater., 2007, 148, 98–102 CrossRef CAS PubMed.
- E. F. Oliveira, Am. Mineral., 2000, 85, 1503–1507 CrossRef.
- J. H. Ramirez, F. J. Maldonado-Hódar, A. F. Pérez-Cadenas, C. Moreno-Castilla, C. A. Costa and L. M. Madeira, Appl. Catal., B, 2007, 75, 312–323 CrossRef CAS.
- D. Tabet, M. Saidi, M. Houari, P. Pichat and H. Khalaf, J. Environ. Manage., 2006, 80, 342–346 CrossRef CAS PubMed.
- H. H. Huang, M. C. Lu and J. N. Chen, Water Res., 2001, 35, 2291–2299 CrossRef CAS PubMed.
- J. C. Barreiro, M. D. Capelato, L. Martin-Neto and H. C. B. Hansen, Water Res., 2007, 41, 55–62 CrossRef CAS PubMed.
- S. Chou, C. Huang and Y. H. Huang, Environ. Sci. Technol., 2001, 35, 1247–1251 CrossRef CAS PubMed.
- W. P. Kwan and B. M. Voelker, Environ. Sci. Technol., 2004, 38, 3425–3431 CrossRef CAS PubMed.
- X. Xue, K. Hanna and N. Deng, J. Hazard. Mater., 2009, 166, 407–414 CrossRef CAS PubMed.
- M. J. Ahmed and S. K. Theydan, Chem. Eng. J., 2013, 214, 310–318 CrossRef CAS.
- B. Pan, J. Wu, B. Pan, L. Lv, W. Zhang, L. Xiao, X. Wang, X. Tao and S. Zheng, Water Res., 2009, 43, 4421–4429 CrossRef CAS PubMed.
- J. Lǚ, H. Liu, R. Liu, X. Zhao, L. Sun and J. Qu, Powder Technol., 2013, 233, 146–154 CrossRef.
- E. W. S. J. S. Han, M. Jang, A. Soohong Min, J. K. Park and R. M. Rowell, Environ. Sci. Technol., 2004, 38, 912–917 CrossRef.
- R. Rahnemaie, T. Hiemstra and W. H. V. Riemsdijk, Langmuir, 2007, 23, 3680–3689 CrossRef CAS PubMed.
- N. Khare, D. Hesterberg and J. D. Martin, Environ. Sci. Technol., 2005, 39, 2152–2160 CrossRef CAS PubMed.
- G. A. Pianetti, L. M. M. D. Campos, P. Chaminade, A. Baillet, D. Baylocq-Ferrier and G. Mahuzier, Anal. Chim. Acta, 1993, 284, 291–299 CrossRef CAS.
- J. Ma and N. J. D. Graham, Water Res., 2000, 34, 3822–3828 CrossRef CAS.
- L. Hou, H. Zhang, L. Wang and C. Lu, Chem. Eng. J., 2013, 229, 577–584 CrossRef CAS.
- B. Boonchom, S. Youngme, S. Maensiri and C. Danvirutai, J. Alloys Compd., 2008, 454, 78–82 CrossRef CAS.
- M. A. G. Aranda, S. Bruque and J. P. Attfield, Inorg. Chem., 1991, 30, 2043–2047 CrossRef CAS.
- A. M. Abdelghany, F. H. ElBatal, H. A. ElBatal and F. M. Ezzeldin, J. Mol. Struct., 2014, 1074, 503–510 CrossRef CAS.
- J. Lu, D. Liu, J. Hao, G. Zhang and B. Lu, Chem. Eng. Res. Des., 2015, 93, 652–661 CrossRef CAS.
- F. Liu, J. Yang, J. Zuo, D. Ma, L. Gan, B. Xie, P. Wang and B. Yang, J. Environ. Sci., 2014, 26, 1751–1762 CrossRef CAS PubMed.
- Q. Yu, Y. Zheng, Y. Wang, L. Shen, H. Wang, Y. Zheng, N. He and Q. Li, Chem. Eng. J., 2015, 260, 809–817 CrossRef CAS.
- Z. Wen, Y. Zhang and C. Dai, Colloids Surf., A, 2014, 457, 433–440 CrossRef CAS.
- K. Nadeem, T. Traussnig, I. Letofsky-Papst, H. Krenn, U. Brossmann and R. Würschum, J. Alloys Compd., 2010, 493, 385–390 CrossRef CAS.
- S. Mitra, S. Das, K. Mandal and S. Chaudhuri, Nanotechnology, 2007, 18, 575–608 CrossRef.
- C. Wang, J. Wu, H. Sun, T. Wang, H. Liu and Y. Chang, Ind. Eng. Chem. Res., 2011, 50, 8515–8523 CrossRef CAS.
- P. Jha and K. Singh, Ceram. Int., 2016, 42, 436–444 CrossRef CAS.
- Z. Li, L. Schulz, C. Ackley and N. Fenske, J. Colloid Interface Sci., 2010, 351, 254–260 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13637k |
| This journal is © The Royal Society of Chemistry 2016 |