Facile fabrication of stable and high-rate Si/NiSix/CNTs Li-ion anodes with a buffering interface†
Abstract
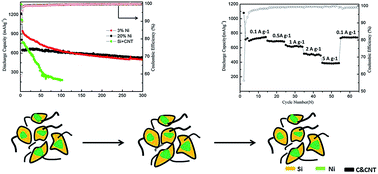
Silicon (Si)/carbon nanotubes (CNTs) composites are ideal anode materials for lithium ion batteries. However, due to the large volume expansion mismatch between Si and CNTs, the cycle life of conventional Si/CNTs composites fabricated by different approaches is still very limited. Here, a novel Si/nickel-Si alloy (NiSix)/CNTs composite has been successfully designed and fabricated through a chemical vapor deposition method with the assistance of uniformly embedded Ni nanoparticles. The CNTs grow up from the composite and serve as strong rivets, which greatly improves the electrical connection stability at the interface between Si and CNTs. Consequently, the corresponding discharge capacity can remain at around 550 mA h g−1 with a capacity retention of 84% for 300 cycles, while a high rate capability can be achieved with a discharge capacity of 420 mA h g−1 at 4 A g−1.

 Please wait while we load your content...
Please wait while we load your content...