Nanostructured BiOI–GO composite: facile room temperature synthesis with enhanced multifunctionality in field emission and photocatalytic activity†
Prashant K. Bankara,
Sambhaji S. Waruleb,
Sandesh R. Jadkara,
Nilima S. Chaudhari*a and
Mahendra A. More*a
aCentre for Advanced Studies in Materials Science and Condensed Matter Physics, Department of Physics, Savitribai Phule Pune University, Pune-411007, India. E-mail: nilima.chaudhari07@gmail.com; mam@physics.unipune.ac.in
bDepartment of Physics, Nowrosjee Wadia College, Savitribai Phule Pune University, Pune-411001, India
First published on 23rd August 2016
Abstract
Coupling of layered semiconductors with graphene-based materials could enable enhanced performance as compared to the pristine counterparts. Herein, we report enhanced multifunctional behaviour of a bismuth oxyiodide–graphene oxide (BiOI–GO) composite regarding its field emission and photocatalytic characteristics. The layered bismuth oxyiodide (BiOI) nanodiscs and nanostructured BiOI–GO composite were synthesized at room temperature employing a facile, single step precipitation method. The as-synthesized products were characterized using XRD, SEM, TEM, and a Raman spectrophotometer so as to reveal their phase, morphological and structural properties. Field emission (FE) studies of pristine layered BiOI nanodiscs and BiOI–GO nanocomposite emitters were carried out at the base pressure of ∼1 × 10−8 mbar. The values of turn on field required to draw an emission current density of 10 μA cm−2 are found to be 2.7 and 1.2 V μm−1 for BiOI nanodiscs and BiOI–GO nanocomposite emitters, respectively. Extraction of an emission current density of ∼1150 μA cm−2 from the BiOI–GO nanocomposite emitter at the remarkably low applied field of 2.8 V μm−1 signifies its enhanced FE performance. The superior FE characteristics of the BiOI–GO nanocomposite emitter are attributed to modulation of the electronic properties due to composite formation, and the high aspect ratio of the nanosheets/nanodiscs. Furthermore, the BiOI–GO nanocomposite exhibits enhanced photocatalytic activity towards degradation of methyl orange (MO) dye.
Introduction
In recent years, intensive research effort has been focused on graphene analogues of inorganic layered materials due to their exotic properties and applications in various nano-electronic devices.1,2 The two dimensional (2D) materials are known for their atomically thin planar structure, and being probed for the development of planar nano-electronic devices.2,3 In this context, graphene, graphene oxide (GO), layered MoS2, WS2, SnS2 etc. have been recently explored by researchers for their FE properties.4–7 Furthermore, graphene based hybrid structures are expected to enhance the FE properties via tuning of the electronic properties of the counterparts.6,7 As seen from the literature, the coupling of layered structures with graphene based materials is mostly limited to the transition metal chalcogenides, enabling future scope for utilization of other layered materials like bismuth oxyhalides. The bismuth oxyhalides (BiOX, X = Cl, Br, I), an important class of V–VI–VII ternary oxide semiconductors, have attracted much attention as a new family of promising photocatalysts.8–10 Such compounds crystallize in the tetragonal structure, a layered structure characterized by [Bi2O2] slabs interleaved by double layers of halogen atoms, resulting into formation of discs-like morphology. Amongst the various bismuth oxyhalides, BiOI has attracted attention for diverse applications in future nano-electronic devices because of layered structure and low direct bandgap.11,12 So far, various synthesis methods have been reported to obtain different morphologies of BiOI such as nanosheets, microspheres.11–13 Furthermore, very recently few researchers have attempted BiOI heterostructures and/or composites.13–17 However, studies on low temperature synthesis of BiOI nanostructures as well as their heterostructures/nanocomposites via a facile approach are sparse in literature. To the best of our knowledge, there is no report on FE characteristics of BiOI nanostructures and their nanocomposites. Therefore, it is of scientific as well as technological importance to undertake systematic studies on low temperature synthesis of BiOI nanostructures and its composite with GO.In the present study, a simple room temperature precipitation method is employed to synthesize BiOI nanodiscs and BiOI–GO nanocomposite, without use of template or surfactant. The morphological, structural and optical properties of the synthesized products have been investigated. The multifunctional behaviour of the BiOI–GO nanocomposite pertaining to field emission and photocatalysis has been investigated. Interestingly, the BiOI–GO nanocomposite exhibits enhanced functionality in comparison to the pristine BiOI sample.
Experimental
Synthesis of layered BiOI nanodiscs
All chemical reagents used in this experimental procedure were of analytical grade and used without further purification. In a typical experiment, 0.02 g KI was dissolved in 20 mL double distilled water (DW), and 0.05 g Bi(NO3)3 was dissolved in 20 mL ethylene glycol, in separate glass beakers. The aqueous KI solution was added drop wise to bismuth nitrate solution and the resulting suspension was stirred rigorously. The suspension exhibited gradual colour change from yellow to red. After continuous stirring for 2 h, the final product was collected via filtration, washed several times with anhydrous ethanol, followed by drying in air at 60 °C for 2 h.Synthesis of BiOI–GO nanocomposite
The BiOI–GO nanocomposite was synthesized following the same precipitation reaction as that of BiOI. In order to synthesize the BiOI–GO nanocomposite with different mass of GO (1, 3 and 5 mg), first appropriate quantity of GO (synthesized as per Hummer's method) was dispersed in 20 mL double distilled water via ultrasonication for 20 min. This GO suspension was added into the bismuth nitrate solution (0.05 g Bi(NO3)3 dissolved in 20 mL ethylene glycol). The rest procedure, same as that employed for synthesis of BiOI, was followed to harvest the final product. A typical reaction process schematic is shown in ESI Scheme S1.† Hereafter, as-synthesized samples name as a BiOI–GO 1 mg (BIGO1), BiOI–GO 3 mg (BIGO2) and BiOI–GO 5 mg (BIGO3).Characterizations
The structural analysis of the as-synthesized samples was carried out using X-ray diffractometer (XRD, D8 advance, Bruker AXS) using Cu Kα line (λ = 1.54056 Å), whereas for morphological studies, a field-emission scanning electron microscope (Hitachi S4800 and Nova nanosem 450) was used. Furthermore, in-depth structural and morphological investigations were performed using transmission electron microscopy (Technai G2 U20 Twin, FEI). The optical properties were investigated using UV-visible-NIR spectrophotometer (Model-JASCO 670). Furthermore, structural characterization was performed by a micro-Raman spectrometer with a laser excitation wavelength of 532 nm with 5 mW laser power in back scattering geometry using LabRam instrument.Field emission
The field emission (FE) current versus applied voltage (I–V) and emission current versus time (I–t) characteristics were measured in a planar ‘diode’ configuration in an all-metal vacuum chamber evacuated to a base pressure of ∼1.0 × 10−8 mbar. In a typical ‘diode’ configuration, a phosphor coated indium tin oxide glass plate (circular disc of diameter ∼50 mm) acts as the anode, whereas the as-synthesized samples (sprinkled onto a piece of ultra high vacuum compatible conducting carbon tape pasted on a Cu rod of diameter 5 mm) served as the cathode. The FE measurements were carried out at a constant cathode–anode separation of ∼2 mm. The emission current was acquired by varying the applied dc voltage between the cathode and anode with a step of 40 V (0–40 kV, Spellman, U.S.). Special care was taken to avoid any leakage current by ensuring proper grounding. The details of vacuum processing and FE measurements are described in detail elsewhere.18Photocatalytic activity
The photocatalytic activity of as-prepared samples was studied via catalytic degradation of methyl orange (MO) in water under visible illumination. A 400 W xenon lamp was used as an illumination source. All experiments were carried out at room temperature. In a typical photocatalysis experiment, 10 mg of photocatalyst (the as-synthesized sample) was dispersed in 100 mL of 5 mg L−1 (5 ppm) MO solution in a quartz reactor. Before irradiation, the dispersed catalyst was magnetically stirred in the dark for 1 h to reach adsorption–desorption equilibrium of dye on the catalyst surface. Then the suspension was subjected to irradiation for definite time intervals. After every 30 minutes, ∼3 mL suspension was collected and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) to remove the photocatalyst particles. The concentration of MO chromophores was estimated from the absorbance at 465 nm on UV-visible spectrophotometer (Model-JASCO 670).
000 rpm, 5 min) to remove the photocatalyst particles. The concentration of MO chromophores was estimated from the absorbance at 465 nm on UV-visible spectrophotometer (Model-JASCO 670).
Results and discussion
The XRD patterns of as-synthesized pristine BiOI and BIGO1 nanocomposite are shown in Fig. 1. At the outset both the diffraction patterns appear very identical exhibiting a set of well defined diffraction peaks, expect variation in intensity of the diffraction peaks. All diffraction peaks (in both the patterns) could be indexed to the tetragonal phase of BiOI (JCPDS-100445), indicating that the presence of GO in the nanocomposite does not affect the crystallinity of BiOI. A careful observation of the XRD pattern of BiOI–GO nanocomposite reveals appearance of a broad hump (12° to 20°), which is indicative of presence of GO in the nanocomposite (see ESI Fig. S1†). No diffraction peaks corresponding to other phases or impurities are observed. Thus, the XRD analysis confirms synthesis of pure crystalline phases under the prevailing experimental conditions.The surface morphology of the as-synthesized products is depicted in Fig. 2. It is apparent that in both cases, the morphology is characterized by nanodiscs like structures of BiOI. In case of pristine BiOI sample (Fig. 2a and b), the assembled BiOI nanostructures are comprised of radially grown nanodiscs of size ∼1–2 μm. The overall morphology exhibited by the BIGO1 nanocomposite (Fig. 2c) is identical to the pristine BiOI sample, indicating no significant change due to presence of GO. The FESEM image recorded at higher magnification (Fig. 2d) reveals presence of large number of protruding edges of the nanodiscs/nanosheets. As both the counterparts, of the nanocomposite, BiOI and GO, possess sheet like morphology, their ‘individual’ identification in the FESEM image (whether the observed nanodiscs is of BiOI or GO) is formidable. The elemental analysis derived from the EDAX spectrum of the nanocomposite sample (depicted in ESI Fig. S2†) clearly indicates formation of BiOI–GO nanocomposite under the prevailing experimental conditions. The observed Si peak in the EDAX spectrum is due to the Si substrate.
In order to gain better understanding of the structural characteristics of the as-synthesized products, transmission electron microscopy (TEM) analysis was carried out. A typical TEM image of the BiOI sample (Fig. 3a) exhibits presence of BiOI nanodiscs with average size ∼500 nm. A careful observation of the high magnification TEM image (Fig. 3b) indicates ‘transparent’ nature of the sheets implying their ultra thinness. Fig. 3c presents the high-resolution (HRTEM) image, obtained at an edge of nanodiscs, reveals crystalline nature of sample. The digitally magnified view of the HRTEM image (inset of Fig. 3c) depicts interplanar spacing of ∼0.28 nm corresponding to the (110) plane of tetragonal phase BiOI. The single crystalline nature of the BiOI nanodiscs is further confirmed by the selected area electron diffraction (SAED) pattern (Fig. 3d). Similarly, for detailed structural investigations of the BIGO1 nanocomposite, TEM and HRTEM analysis was carried out. A typical low-magnification TEM image (Fig. 4a) is reveals the presence of both GO nanosheets and BiOI nanodiscs. Interestingly, the GO sheets (Fig. 4b) are not very flat but display intrinsic wrinkles. The high-resolution TEM (HRTEM) image deliberately taken at the contact edge of BiOI nanodiscs and GO nanosheet reveals the crystalline nature of the nanocomposite. From an inset of Fig. 4c, the spacing between adjacent lattice fringe is estimated to be ∼0.28 nm, which is close to the d spacing of the (110) plane. The SAED pattern (Fig. 4d) supports the formation of single crystalline phases of both the BiOI nanodiscs and the GO nanosheet.
 | ||
| Fig. 3 TEM images of the BiOI nanodiscs at (a) low and (b) high magnification, (c) HRTEM images of BiOI nanodiscs, and (d) selected area electron diffraction (SAED) pattern of BiOI nanodiscs. | ||
Raman spectroscopy has important role in the structural characterization of carbon based materials. A typical or standard Raman spectrum of graphite oxide shows two prominent signatures at 1350 and 1570 cm−1, ascribed as D and G bands, respectively. The G band is generally assigned to the E2g phonons of sp2 carbon atom; whereas the D band is attributed to the local defect and disorders.19 Fig. 5 depicts the Raman spectra of BIGO1 nanocomposite and pristine BiOI samples. Raman spectra of BiOI exhibit an intense peak at 151.6 cm−1 which assigned to the Eg internal Bi–X stretching mode.20 The Raman spectrum of the nanocomposite shows signatures of both the counterparts, BiOI and GO in terms of appearance of peaks at 151.6, 1340 and 1596 cm−1. The shift in the peak positions of G and D bands in the BOI–GO spectrum, with reference to pristine GO sample, is indicative of the formation of composite under the prevailing experimental conditions. In addition to this, we have also performed the Raman spectroscopy studies of the BIGO2 and BIGO2 samples (please see ESI Fig. S3†). The Raman spectra of these samples indicate spectral features identical to that of BIGO1 sample, except change in the intensities of the observed peaks. Furthermore, in order to understand the details of optical properties of as-synthesized materials; UV-vis spectroscopy and PL were carried out (see ESI Fig. S4 and S5†). Such spectral of features has been explained by H. Liu and co-workers.15
Field emission
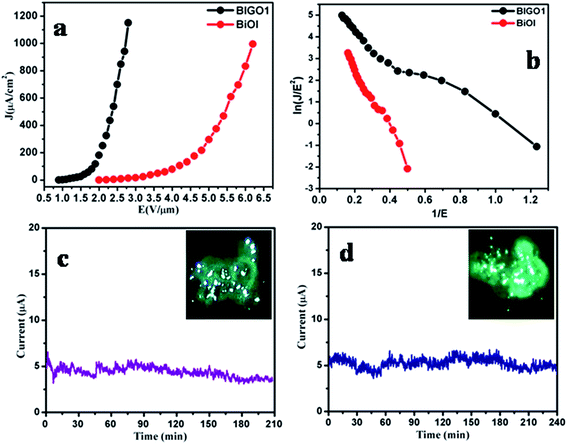
Fig. 6 shows the field emission current density versus applied electric field (J–E) characteristics of BiOI nanodiscs and BIGO1 nanocomposite emitters. With the increase in applied field the emission current increases exponentially, indicating that the emission of electrons is as per the Fowler–Nordheim (F–N) theory.21,22 The modified form of the Fowler–Nordheim equation is given as,
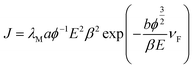 | (1) |
Photocatalytic activity
The photocatalytic activity of as-prepared BiOI and BiOI–GO nanocomposite samples towards the degradation of methyl orange (MO) was investigated under visible illumination. As the degradation of MO occurs via three different routes i.e. photolysis, photosensitization of dye and photocatalytic process, we have confirmed the photocatalytic degradation by ‘dark’ reaction study. We observed that there is no change in the concentration of MO chromophores under dark conditions over longer duration more than 8 h. Hence, in this study the degradation of MO occurs via photocatalytic process. In a detailed study, we have compared the photocatalytic activities of pristine BiOI, and the BIGO1, BIGO2 and BIGO3 samples (Fig. 7 and ESI Fig. S7†). It is observed that the BIGO1 nanocomposite shows 82% decomposition of MO while pristine BiOI shows 71% decomposition after 150 min. Interestingly, after 90 min irradiation on BIGO1 catalyst system, 78% loss of MO has been observed and which further saturates after 180 min. Apart from this, in case of pristine BiOI system after 90 min irradiation, only 52% loss of MO is observed. From this result, we can say that the BIGO1 nanocomposite exhibits enhanced photocatalytic activity towards degradation of MO dye. It is well known that the cycle stability/photostability of a catalyst is an important factor from its practical application point of view. Hence, we have also carried out the stability study of BIGO1 sample by reusing the sample for few cycles. This data is shown in ESI Fig. S6.† The nature of graph clearly indicates that, this nanocomposite material possesses very high photostability. | ||
| Fig. 7 Photocatalytic activities of BiOI and BiOI–GO samples on the degradation of MO under visible light irradiation. | ||
As an extension to this work, we have also studied the effect of GO concentrations (in BIGO nanocomposites) on the photocatalytic properties. In this study it is observed that, the BIGO2 nanocomposite shows slightly lower photocatalytic activity than the BIGO1 sample. Furthermore, the BIGO3 nanocomposite exhibits lowest photocatalytic activity amongst the three nanocomposites, (please see ESI Fig. S7†). Instead of degradation of MO, this sample shows dye adsorption and desorption phenomenon due to the presence of excessive amount of GO with high surfaces area. This clearly indicate that BIGO1 is the nanocomposite with optimal concentration of GO so as to synthesize BIGO nanocomposite possessing highest photocatalytic activity for MO degradation.
It is well known that the generation of electron–hole (e−–h+) pairs and their separation over the catalyst surface are important steps in photocatalytic reactions. In case of pristine BiOI sample, although the rate of e−–h+ pair generation under visible illumination is noticeable (due to its narrow band gap), their separation rate is poor resulting into more recombination of e−–h+ pairs. Thus, the photocatalytic degradation of MO takes more time or is a slower process. In order to enhance the photocatalytic activity of BiOI, the e−–h+ pairs should be effectively separated before recombination, and used for accelerating the MO degradation reactions. This is achieved via formation of BIGO1 composites, wherein due to the formation of heterostructure the rate of e−–h+ pair generation and separation are apparently same. The detailed mechanism of enhancement of photocatalytic activity has been discussed by Hong Liu and co-workers.15,23 The enhanced photocatalytic activity can be attributed to more effective charge transportations and separations arisen from the strong chemical bonding between BiOI and GO, the high dye adsorption performance, and the increased light absorption.
On the other hand, amount of GO is also an important factor in photocatalytic study. In this study, 10 mg is an optimal amount of GO required to obtain highest photocatalytic activity of BiOI. Further increase in the concentration of GO (30 mg and 50 mg) is causes deterioration in photocatalytic activity. Because, excessive GO acts as a charge (e−–h+) recombination centers. Also instead of BiOI, excessive GO starts to absorb visible light from the source and leads to decrease in the photocatalytic activity.15
Conclusion
In nutshell, template free room temperature co-precipitation method is effectively employed to synthesize BiOI nanodiscs and nanostructured BiOI–GO composite. The tetragonal-phase and single crystalline nature of BiOI nanodiscs was confirmed using XRD and HRTEM, respectively. Electron microscopy reveals the layered BiOI nanodiscs blending with suspension of GO and finally BiOI nanodiscs is effectively reinforce with GO nanosheets. The as-synthesized BiOI–GO nanocomposite demonstrated enhanced field emission and photocatalytic activity in comparison to pristine materials. The values of turn on field, required to draw emission current density of 10 μA cm−2, are found to be 2.7 and 1.2 V μm−1 for BiOI nanodiscs and BiOI–GO nanocomposite emitters, respectively. Extraction of emission current density of ∼1150 μA cm−2 from the BiOI–GO nanocomposite emitter at remarkably low applied field of 2.8 V μm−1 signifies its enhanced FE performance. Also, the BiOI–GO nanocomposite exhibits enhanced photocatalytic activity towards degradation of MO dye. The present approach of coupling layered bismuth oxyhalides with graphene-based materials nanocomposite may be exploited to enhance other functionalities.Acknowledgements
P. K. Bankar acknowledges SPPU for the financial support. Prof. M. A. More would like to thank the BCUD, of Savitribai Phule Pune University for the financial support provided for the field emission work under CNQS-UPE-UGC program activity. Dr N. S. Chaudhari gratefully acknowledges the financial support from UGC India under the award of Dr D. S. Kothari postdoctoral fellowship (BSR/CH/13-1) (BSR/CH/13-14/0102).Notes and references
- C. N. R. Rao, H. S. S. Ramakrishna Matte and U. Maitra, Angew. Chem., Int. Ed., 2013, 52, 13162–13185 CrossRef CAS PubMed.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- T. Zhai, L. Li, Y. Ma, M. Liao, X. Wang, X. Fang, J. Yao, Y. Bando and D. Golberg, Chem. Soc. Rev., 2011, 40, 2986–3004 RSC.
- D. Ye, S. Moussa, J. D. Ferguson, A. A. Baski and M. S. El-Shall, Nano Lett., 2012, 12, 1265–1268 CrossRef CAS PubMed.
- R. V. Kashid, D. J. Late, S. S. Chou, Y.-K. Huang, D. S. Joag, M. A. More and V. P. Dravid, Small, 2013, 9, 2730–2734 CrossRef CAS PubMed.
- C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Sci. Rep., 2013, 3, 3282 Search PubMed.
- C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Appl. Phys. Lett., 2014, 105, 043109-5 CrossRef.
- D. S. Bhachu, S. J. A. Moniz, S. Sathasivam, D. O. Scanlon, A. Walsh, S. M. Bawaked, M. Mokhtar, A. Y. Obaid, I. P. Parkin, J. Tang and C. J. Carmalt, Chem. Sci., 2016, 7, 4832–4841 RSC.
- J. Li, Y. Yu and L. Zhang, Nanoscale, 2014, 6, 8473–8488 RSC.
- A. M. Ganose, M. Cuff, K. T. Butler, A. Walsh and D. O. Scanlon, Chem. Mater., 2016, 28, 1980–1984 CrossRef CAS PubMed.
- Z. Y. Zhao and W. W. Dai, Inorg. Chem., 2015, 54, 10732–10737 CrossRef CAS PubMed.
- C. Chen, P. Hu, X. Hu, Y. Meia and Y. Huang, Chem. Commun., 2015, 51, 2798–2801 RSC.
- X. Xiao, R. Hao, M. Liang, X. Zuo, J. Nan, L. Li and W. Zhang, J. Hazard. Mater., 2012, 233, 122–130 CrossRef PubMed.
- J. Jiang, X. Zhang, P. Sun and L. Zhang, J. Phys. Chem. C, 2011, 115, 20555–20564 CAS.
- H. Liu, W. R. Cao, Y. Su, Z. Chen and Y. Wang, J. Colloid Interface Sci., 2013, 398, 161–167 CrossRef CAS PubMed.
- S. Y. Chou, C. C. Chen, Y. M. Dai, J. H. Lin and W. W. Lee, RSC Adv., 2016, 6, 33478–33491 RSC.
- J. C. Wang, H. C. Yao, Z. Y. Fan, L. Zhang, J. S. Wang, S. Q. Zang and Z. J. Li, ACS Appl. Mater. Interfaces, 2016, 8, 3765–3775 CAS.
- N. S. Ramgir, D. J. Late, A. B. Bhise, I. S. Mulla, M. A. More, D. S. Joag and V. K. Pillai, Nanotechnology, 2006, 17, 2730–2735 CrossRef CAS.
- K. Krishnamoorthy, M. Veerapandian, R. Mohan and S. Kimes, Appl. Phys. A: Mater. Sci. Process., 2012, 106, 501–506 CrossRef CAS.
- S. Bunda and V. Bunda, Acta. Phys. Pol., 2014, 126, 272–273 CrossRef.
- R. G. Forbes, J. Appl. Phys., 2008, 103, 114911 CrossRef.
- R. G. Forbes, Appl. Phys. Lett., 2006, 89, 113122 CrossRef.
- L. Ye, J. Fu, Z. Xu, R. Yuan and Z. Li, ACS Appl. Mater. Interfaces, 2014, 6, 3483 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of XRD spectra, EDS spectra, Raman, UV-vis, photoluminescence spectra, photocatalytic activity and stability. See DOI: 10.1039/c6ra13471h |
| This journal is © The Royal Society of Chemistry 2016 |