Solvent-free one step aminolysis and alcoholysis of low-quality triglycerides using sodium modified CaO nanoparticles as a solid catalyst†
Dinesh Kumar *a,
Soo Min Kimb and
Amjad Ali*c
*a,
Soo Min Kimb and
Amjad Ali*c
aDepartment of Bionanosystem Engineering, Chonbuk National University, Jeonju-561-756, South Korea. E-mail: dineshkumar@jbnu.ac.kr; dinesh.tu@gmail.com
bDepartment of BIN-Fusion Technology, Chonbuk National University, Jeonju-561-756, South Korea
cSchool of Chemistry and Biochemistry, Thapar University, Patiala-147004, India. E-mail: amjadali@thapar.edu
First published on 6th June 2016
Abstract
Here, we report the solvent-free one step preparation of fatty acid amides and fatty acid alkyl esters from low quality triglycerides using a solid catalyst. A series of Na+ modified CaO has been prepared in the form of nanoparticles by a modified incipient-wetness impregnation method, and used as a solid catalyst for aminolysis and alcoholysis of triglycerides. The prepared catalyst 3-NaC/CaO-450 (3 wt% Na impregnated in CaO) was found to exist in nanoparticle form (particle size = 25 nm) as suggested by TEM, DLS and powder XRD studies, have a surface area of 10.9 m2 g−1 (BET method) and found to have the basic strength of 15.0 < H_ < 18.4 as supported by the Hammett indicator test. The catalyst was found to complete the aminolysis (5 wt% catalyst; 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of diethanolamine/oil; 110 °C) with excellent recyclability and is also equally efficient for the alcoholysis (5 wt% catalyst; 12
1 molar ratio of diethanolamine/oil; 110 °C) with excellent recyclability and is also equally efficient for the alcoholysis (5 wt% catalyst; 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol/oil; 65 °C) of used cotton seed oil.
1 molar ratio of methanol/oil; 65 °C) of used cotton seed oil.
1. Introduction
Renewable raw materials are going to play a significant role for upgrading sustainable green chemistry. Renewable raw materials viz., vegetable oils and fat share the great proportion of the current consumption in the chemical industry.1 The derivatives of vegetable oils have numerous benefits viz., they are renewable, nontoxic, biodegradable and environmentally friendly. These derivatives have potential industrial applications such as fatty acid methyl esters (FAMEs) which are a desired substitute for fossil fuels2 and fatty acid amides (FAA) and have attracted attention due to their biological activities and industrial applications in surfactants, lubricants, cosmetics, shampoos, foam control agents, fungicides, corrosion inhibitors, water repellents and detergents.3–5 Due to low reactivity and thermal properties they are also used for the synthesis of anti-block and anti-slip additives in polyethylene films.6 Fatty acid amides also considered to increase the ignition quality characteristic of the fuel as it increases the cetane number of the fuel.7,8FAMEs mostly obtained by the alcoholysis (transesterification) reaction of the naturally occurring triglycerides (vegetable oils or animal fat) with short chain alcohols (e.g., MeOH and EtOH) in the presence of homogeneous catalysts (for example NaOH, KOH, NaOMe, KOMe, HCl and H2SO4)9–11 or heterogeneous catalysts (for example Li/CaO, Zn/CaO).12,13
On the other hand due to their numerous applications, fatty acid amides produced at industrial scale from fatty acids by reaction with anhydrous ammonia at high temperature (∼200 °C) and high pressure (345–690 kPa).14 Fatty acid amides can also be prepared from fatty acids or fatty acid alkyl esters derived from fatty acids through treatments with different amine compounds.15,16 Enzymatic catalyzed amidation from the fatty acids or their esters has also been reported.14,17,18 Triglycerides or vegetable oils can be converted into their fatty acid amides via amidation using ammonia or amine compounds.19,20 The fatty acid amides were prepared by refluxing palm oil and urea using sodium ethoxide as a homogeneous catalyst in the presence of ethanol.21 Monosubstituted fatty acid amides were synthesized by reacting primary amine with vegetable oil, tallow, and fish oil at the boiling point of amine for longer reaction time.22,23 Ethylenediamine has also been used for the preparation of fatty acid amides by using fatty acids at 180–185 °C under nitrogen environment and with continuous removal of water.24 However, high temperature and long reaction time causes self-condensation of diethylamine that results to form N,N′-bis(2-hydroxyethyl) piperazine or morpholine instead of FAA.25,26
The preparation processes of FAMEs and FAA utilized mostly the homogeneous catalysts, however, homogeneous catalysts have several disadvantages like, non-recyclability, contaminated product and deactivation by high moisture (>0.3 wt%) and free fatty acid (>0.5 wt%) contents in feedstock.27,28 Also, the use heterogeneous catalyst especially in case of FAA synthesis, such as lipase, require high temperature and high pressure. Thus, the whole process become tedious, lengthy and requires large amounts of water and energy. To overcome these drawbacks, solid catalysts could be more advantageous as these are reusable, less corrosive, easy to separate from the reaction mixture27,29 and effective even when moisture and FFA contents are high in feedstocks.12
Present study demonstrated the preparation of Na+ modified CaO in nano-particle form and its characterization by powder XRD, SEM, TEM, Hammett indicators and surface area measurement studies. The prepared catalyst has been used as solid catalyst and found as fully efficient for the one step solvent free aminolysis and alcoholysis of used cotton seed oil with diethanolamine (DEA) and methanol, respectively.
2. Experimental section
2.1 Materials and methods
The chemicals used in present study viz., sodium carbonate, lithium carbonate, potassium carbonate, calcium oxide, methanol (99.8%), methyl laurate (>98%) and diethanolamine (99.8%) were purchased from Sigma-Aldrich, USA.The free fatty acids (FFAs) value, iodine, and the saponification value of the mutton fat (MF), soybean oil (SO), virgin cotton seed (CSO), used cottonseed seed oil (UCSO), castor oil (CO), karanja oil (KO), and jatropha oil (JO) were determined by following the methods as reported in literature30 and the moisture content was determined by the Karl Fisher titrimetric method (Table 1).
| S. No. | Feedstock | Free fatty acid value (wt%) | Moisture content (wt%) | Saponification value (mg KOH per g) | Iodine value |
|---|---|---|---|---|---|
| 1 | Used cotton seed oil | 2.2 | 0.40 | 190.3 | 101.3 |
| 2 | Karanja oil | 4.1 | 0.35 | 184 | 96.6 |
| 3 | Jatropha oil | 8.4 | 0.38 | 195.2 | 101.3 |
| 4 | Virgin cotton seed oil | 0.1 | 0.31 | 188.4 | 102.4 |
| 5 | Soybean oil | 0.2 | 0.27 | 189.6 | 125.1 |
| 6 | Mutton fat | 1.3 | 0.32 | 192.1 | 46.3 |
| 7 | Castor oil | 2.0 | 0.34 | 180.2 | 90.5 |
2.2 Preparation of catalyst
The modified incipient-wetness impregnation method31 was employed to prepare nanocrystalline sodium metal ion impregnated calcium oxide. In a typical preparation, CaO slurry (10 mg/40 mL ethanol) was sonicated for 1 h then 10 mL of alkali metal (Na2CO3 or Li2CO3 or K2CO3) solution in ethanol of desired concentration was added drop wise into CaO slurry and stirred for 2 h at room temperature. The concentration of the sodium carbonate was also varied to obtain Na+ loading in the range of 1 to 5 wt% in CaO. The mixture was stirred for 3 h, then dried and calcined at varying temperature of 250–650 °C for 12 h. The solid thus obtained was characterized by powder XRD, BET surface area measurement, Hammett indicator test, DLS, SEM and TEM techniques.The prepared catalysts were designated as xx-NaC/CaO-T, where xx represents the sodium concentration (wt%) in CaO and T represents calcinations temperature. For example 3-NaC/CaO-450 represent the catalyst prepared by impregnating 3 wt% of Na+ (using Na2CO3) in CaO and calcined at 450 °C.
2.3 Aminolysis reactions
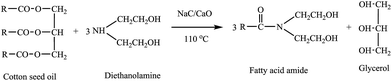
Aminolysis reactions of used cotton seed oil, mutton fat or methyl laurate and FAMEs derived from used cotton seed oil and mutton fat have been performed with diethanolamine (DEA) in the presence of 3-NaC/CaO-450 catalyst as shown in Schemes 1–3. All aminolysis reactions were performed in a 100 mL two necks round bottom flask fitted out with a water-cooled condenser, magnetic stirrer and oil bath. Vegetable oil was mixed with diethanolamine to maintain the diethanolamine to oil molar ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in the presence of 5 wt% catalyst (3-NaC/CaO-450) at 110 °C in a typical aminolysis reaction as shown in Scheme 1. Aminolysis reactions of used cotton seed oil have been carried out by varying one parameter at a time in order to institute the best suited reaction conditions for the complete aminolysis. After the finishing point of the reaction, the final reaction mixture was filtered through ordinary filter paper and was dissolved in 60 mL hexane and placed in a separatory funnel. The lighter upper hexane layer was separated from the heavier lower glycerol layer, washed with water, dried with sodium sulfate. Finally, the hexane was rotary-evaporated to yield the pure fatty acid amide (FAA) derivative.
1, in the presence of 5 wt% catalyst (3-NaC/CaO-450) at 110 °C in a typical aminolysis reaction as shown in Scheme 1. Aminolysis reactions of used cotton seed oil have been carried out by varying one parameter at a time in order to institute the best suited reaction conditions for the complete aminolysis. After the finishing point of the reaction, the final reaction mixture was filtered through ordinary filter paper and was dissolved in 60 mL hexane and placed in a separatory funnel. The lighter upper hexane layer was separated from the heavier lower glycerol layer, washed with water, dried with sodium sulfate. Finally, the hexane was rotary-evaporated to yield the pure fatty acid amide (FAA) derivative.
In order to monitor the continuous progress of the reaction, the samples from the reaction mixture have been withdrawn after every 10 min, and subjected to FTIR analysis.
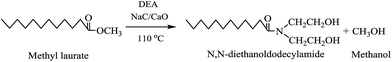
To test the efficacy of NaC/CaO catalyst with other substrates, same catalyst has also been employed for catalyzing the aminolysis reactions of used cotton seed oil derived FAMEs (Scheme 2) and commercially available methyl laurate (Scheme 3) using diethanolamine to FAMEs or methyl laurate molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the presence of 5 wt% catalyst (NaC/CaO) at 110 °C.
1 in the presence of 5 wt% catalyst (NaC/CaO) at 110 °C.
The reaction mixture was filtered through ordinary filter paper after the completion of the reaction, washed with water and organic layer was dried over sodium sulphate. The FAA so obtained was further characterized by FTIR (Fig. S2, ESI†) and 1H-NMR (Fig. S3, ESI†) techniques. The amide product obtained from the amidation of the methyl laurate was also characterized by mass spectrometry (Fig. S4, ESI†) besides FTIR and proton NMR studies. The FTIR technique has also been used to monitoring of reaction and quantification of the amide products formed by following the literature reported procedure.7
FAA derived from used cotton seed oil: yield > 99%. FTIR (cm−1): 3397 (νOH), 1615 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (CDCl3, δ ppm): 5.3 (m, –CH
O); 1H-NMR (CDCl3, δ ppm): 5.3 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.77 (m, –CH2OH), 3.46 (m, –NCH2–), 2.7 (m, –CH
CH–), 3.77 (m, –CH2OH), 3.46 (m, –NCH2–), 2.7 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.31 (m, –CH2–CO–), 2.0 (m, –CH2–(CH2)n–CH–), 1.6–1.25 (m, –(CH2)n–), 0.95 (m, –CH
CH–), 2.31 (m, –CH2–CO–), 2.0 (m, –CH2–(CH2)n–CH–), 1.6–1.25 (m, –(CH2)n–), 0.95 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3), 0.87 (m, –CH2–CH3).
CH–CH3), 0.87 (m, –CH2–CH3).
FAA derivative of used cotton seed oil derived FAMEs: yield > 99%. FTIR (cm−1): 3398 (νOH), 1615 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (CDCl3, δ ppm): 5.3 (m, –CH
O); 1H-NMR (CDCl3, δ ppm): 5.3 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.78 (m, –CH2OH), 3.48 (m, –NCH2–), 2.7 (m, –CH
CH–), 3.78 (m, –CH2OH), 3.48 (m, –NCH2–), 2.7 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.3 (m, –CH2–CO–), 2.0 (m, –CH2–(CH2)n–), 1.6–1.25 (m, –(CH2)n–), 0.95 (m, –CH
CH–), 2.3 (m, –CH2–CO–), 2.0 (m, –CH2–(CH2)n–), 1.6–1.25 (m, –(CH2)n–), 0.95 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3), 0.87 (m, –CH2–CH3).
CH–CH3), 0.87 (m, –CH2–CH3).
FAA derivative of mutton fat derived FAMEs: yield > 99%. FTIR (cm−1): 3398 (νOH), 1615 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (CDCl3, δ ppm): 5.3 (m, –CH
O); 1H-NMR (CDCl3, δ ppm): 5.3 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.75 (m, –CH2OH), 3.47 (m, –NCH2–), 2.3 (m, –CH2–CO–), 2.0 (m, –CH2–(CH2)n–), 1.6–1.25 (m, –(CH2)n–), 0.87 (m, –CH2–CH3).
CH–), 3.75 (m, –CH2OH), 3.47 (m, –NCH2–), 2.3 (m, –CH2–CO–), 2.0 (m, –CH2–(CH2)n–), 1.6–1.25 (m, –(CH2)n–), 0.87 (m, –CH2–CH3).
FAA derivative of methyl laurate (N,N-diethanoldodecylamide): yield > 99%. FTIR (cm−1): 3397 (νOH), 1615 (νamide–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1H-NMR (CDCl3, δ ppm): 3.8 (m, –CH2OH), 3.5 (m, –NCH2–), 2.3 (m, –CH2–CO–), 1.6–1.25 (m, –(CH2)n–), 0.87 (m, –CH2–CH3); EI-MS (m/z) (intensity (%), fragment): 287.2 (3, M), 270.3 (100, M–H2O), 227.22 (10, M–CH3(CH2)3), 175.2 (10, M–CH3(CH2)7), 132.2 (12, M–CH3(CH2)10), 114.2 (5, M–CH3(CH2)10OH).
O); 1H-NMR (CDCl3, δ ppm): 3.8 (m, –CH2OH), 3.5 (m, –NCH2–), 2.3 (m, –CH2–CO–), 1.6–1.25 (m, –(CH2)n–), 0.87 (m, –CH2–CH3); EI-MS (m/z) (intensity (%), fragment): 287.2 (3, M), 270.3 (100, M–H2O), 227.22 (10, M–CH3(CH2)3), 175.2 (10, M–CH3(CH2)7), 132.2 (12, M–CH3(CH2)10), 114.2 (5, M–CH3(CH2)10OH).
2.4 Alcoholysis reaction
In order to show the effectiveness of the 3-NaC/CaO-450 for catalyzing the reactions other than aminolysis, same catalyst has also been used for the transesterification or alcoholysis of variety of triglycerides (virgin cotton seed oil, used cotton seed oil, soybean oil, castor oil, karanja oil, jatropha oil and mutton fat) with methanol as shown in Scheme 4. All transesterification reactions were carried out in a 100 mL, two neck round bottom flask fitted with a water-cooled condenser, oil bath and a magnetic stirrer. In a typical transesterification reaction, triglyceride was mixed with methanol (in 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with respect to triglyceride) with 5 wt% of 3-NaC/CaO-450 and heated at 65 °C, till the completion of reaction.
1 molar ratio with respect to triglyceride) with 5 wt% of 3-NaC/CaO-450 and heated at 65 °C, till the completion of reaction.
The reaction mixture was filtered to remove the catalyst after the completion of the reaction, rotary evaporated to recover the excess methanol and finally kept in separating funnel for 12 h to separate the lower glycerol layer from upper FAMEs layer. FAMEs thus obtained, were further analyzed and quantified by 1H-NMR (Fig. S5, ESI†) by following the literature reported procedure12 as given below:
| Yield = {2I(methoxy)/3I(methylene)} × 100 |
FAMEs derived from used cotton seed oil: H-NMR (CDCl3, δ ppm): 5.31 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.6 (s, –OCH3), 2.7 (m, –CH
CH–), 3.6 (s, –OCH3), 2.7 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.31 (m, –CH2–CO–), 2.0 (m, –CH2–CH
CH–), 2.31 (m, –CH2–CO–), 2.0 (m, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 1.6–1.25 (m, –(CH2)n–), 0.94 (m, –CH
CH–), 1.6–1.25 (m, –(CH2)n–), 0.94 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3), 0.86 (m, –CH2–CH3).
CH–CH3), 0.86 (m, –CH2–CH3).
FAMEs derived from karanja oil: 1H-NMR (CDCl3, δ ppm): 5.33 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.6 (s, –OCH3), 2.72 (m, –CH
CH–), 3.6 (s, –OCH3), 2.72 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.3 (m, –CH2–CO–), 2.01 (m, –CH2–(CH2)n–), 1.61–1.25 (m, –(CH2)n–), 0.96 (m, –CH
CH–), 2.3 (m, –CH2–CO–), 2.01 (m, –CH2–(CH2)n–), 1.61–1.25 (m, –(CH2)n–), 0.96 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3), 0.87 (m, –CH2–CH3).
CH–CH3), 0.87 (m, –CH2–CH3).
FAMEs derived from jatropha oil: 1H-NMR (CDCl3, δ ppm): 5.3 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.61 (s, –OCH3), 2.73 (m, –CH
CH–), 3.61 (s, –OCH3), 2.73 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.31 (m, –CH2–CO–), 2.03 (m, –CH2–(CH2)n–), 1.61–1.25 (m, –(CH2)n–), 0.97 (m, –CH
CH–), 2.31 (m, –CH2–CO–), 2.03 (m, –CH2–(CH2)n–), 1.61–1.25 (m, –(CH2)n–), 0.97 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3), 0.85 (m, –CH2–CH3).
CH–CH3), 0.85 (m, –CH2–CH3).
FAMEs derived from soybean oil: 1H-NMR (CDCl3, δ ppm): 5.31 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.6 (s, –OCH3), 2.76 (m, –CH
CH–), 3.6 (s, –OCH3), 2.76 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH2–CH
CH–CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.3 (m, –CH2–CO–), 2.04 (m, –CH2–(CH2)n–), 1.6–1.25 (m, –(CH2)n–), 0.98 (m, –CH
CH–), 2.3 (m, –CH2–CO–), 2.04 (m, –CH2–(CH2)n–), 1.6–1.25 (m, –(CH2)n–), 0.98 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3), 0.88 (m, –CH2–CH3).
CH–CH3), 0.88 (m, –CH2–CH3).
FAMEs derived from castor oil: 1H-NMR (CDCl3, δ ppm): 6.48 (–OH) 5.52 (m, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 5.38 (m, –CH
CH–), 5.38 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.6 (s, –OCH3), 3.61 (m, –CH–OH), 2.31 (m, –CH2–CO–), 2.21 (m, –CH2–CH
CH–), 3.6 (s, –OCH3), 3.61 (m, –CH–OH), 2.31 (m, –CH2–CO–), 2.21 (m, –CH2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 2.02 (m, –CH2–(CH2)n–), 1.61–1.30 (m, –(CH2)n–), 0.97 (m, –CH
CH–), 2.02 (m, –CH2–(CH2)n–), 1.61–1.30 (m, –(CH2)n–), 0.97 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH3), 0.87 (m, –CH2–CH3).
CH–CH3), 0.87 (m, –CH2–CH3).
FAMEs derived from mutton fat: 1H-NMR (CDCl3, δ ppm): 5.35 (m, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 3.6 (s, –OCH3), 2.31 (m, –CH2–CO–), 2.03 (m, –CH2–(CH2)n–), 1.61–1.25 (m, –(CH2)n–), 0.86 (m, –CH2–CH3).
CH–), 3.6 (s, –OCH3), 2.31 (m, –CH2–CO–), 2.03 (m, –CH2–(CH2)n–), 1.61–1.25 (m, –(CH2)n–), 0.86 (m, –CH2–CH3).
3. Results and discussion
3.1 Catalyst preparation and characterization
The surface area of commercially available calcium oxide and 3-NaC/CaO-450 was measured by BET method and the same was found to be increased from 3.9 m2 g−1 to 10.9 m2 g−1 after impregnating the CaO with 3 wt% of sodium as shown in Table 2. The calcinations temperature has direct impact on surface area and it was found to increase from 8.6 m2 g−1 to 10.9 m2 g−1 as calcinations temperature increases from 250 to 450 °C. Although, further increase in calcinations temperature (650 °C) reduces surface area to 7.8 m2 g−1 which could be due to the sintering of particles. The impregnation of lithium and potassium also caused the increase in surface area from 3.9 to 9.5 and 9.6, respectively.
| S. No. | Catalyst type | BET surface area (m2 g−1) | Particle size (Debye Scherrer) nm | Basic strength (H_) |
|---|---|---|---|---|
| 1 | CaO | 3.9 | 105 | 9.8 < H_ < 10.1 |
| 2 | 1-NaC/CaO-450 | 10.8 | 32 | 10.1 < H_ < 11.1 |
| 3 | 2-NaC/CaO-450 | 10.7 | 28 | 11.1 < H_ < 15.0 |
| 4 | 3-NaC/CaO-450 | 10.9 | 28 | 15.0 < H_ < 18.4 |
| 5 | 4-NaC/CaO-450 | 10.8 | 27 | 15.0 < H_ < 18.4 |
| 6 | 5-NaC/CaO-450 | 10.9 | 29 | 15.0 < H_ < 18.4 |
| 7 | 3-NaC/CaO-250 | 8.6 | 35 | 11.1 < H_ < 15.0 |
| 8 | 3-NaC/CaO-650 | 7.8 | 41 | 11.1 < H_ < 15.0 |
| 9 | 3-LiC/CaO-450 | 9.5 | 70 | 15.0 < H_ < 18.4 |
| 10 | 3-KC/CaO-450 | 9.6 | 39 | 15.0 < H_ < 18.4 |
The absence of the diffraction patterns of Na2CO3, Li2CO3 and K2CO3 could be either due to its high degree of dispersion on the CaO surface. Debye–Scherrer method33 was used for the particle size determination of all the prepared catalysts using powder XRD data. Particle sizes of the 3-NaC/CaO-450 (28 nm) were found to be significantly lower than that of pure CaO (105 nm) as shown in Table 1. The change of Na+ concentration (1–5 wt%) in CaO was not found to alter the structure (Fig. S1, ESI†) and particle size of NaC/CaO significantly (Table 2). The decrease in the particle size and presence of both oxide (CaO) and hydroxide moieties (Ca(OH)2) could lead to higher activity of 3-NaC/CaO-450 towards the aminolysis and transesterification reactions in comparison to pure CaO.
 | ||
| Fig. 2 (A) SEM, and (B) TEM images of 3-NaC/CaO-450. (C) Particle size distributions of CaO and (D) 3-NaC/CaO-450. | ||
The characterization studies of 3-NaC/CaO-450 reveals that it is not only exist in nano particle form but also possess higher basic strength and larger surface area than pure CaO and hence, expected to show higher activity.
3.2 Aminolysis reaction
The prepared alkali metal impregnated CaO has been used as catalyst for the aminolysis of a variety of feedstocks (cotton seed oil, used cotton seed oil, mutton fat, methyl laurate, and FAMEs derived from used cotton seed oil and mutton fat) with diethanolamine. Meanwhile, used cotton seed oil (2.2 wt% FFAs) has been selected for optimizing the parameters to achieve the complete aminolysis.In order to determine the optimum calcination temperature for the better catalytic activity, 3 wt% Na doped CaO was calcined in the temperature range of 250–650 °C. The reaction time for complete conversion (98 ± 2%) to FAA was found to decrease from 1 h to 0.5 h with the increase in calcination temperature of NaC/CaO from 250 to 450 °C. Moreover, NaC/CaO at 450 °C calcination temperature has higher values of BET surface area and basic strength, thus allowing a higher concentration of Lewis base at the catalyst surface. However, a further increase in calcination temperature (650 °C) was found to increase the reaction time to 0.75 h for complete conversion to FAA yield as shown in Fig. 3B. The lesser activity at higher calcination temperature (>450 °C) could be attributed to the reduction in surface area as well as basic strength of the catalyst due to the sintering of the NaC/CaO particles (Table 2). The turnover frequency was found to be maximum when calcinations temperature was 450 °C (39.6 h−1) and also suggested that 450 °C is optimum calcinations temperature for better rate of reaction. Thus 3 wt% Na doped CaO calcined at 450 °C (3-NaC/CaO-450) was found to be more efficient than its other counterparts, and hence, employed for optimizing the reaction parameters for the aminolysis and transesterification of UCSO.
To determine the optimal sodium ion concentration in CaO to achieve the best catalytic activity, a series of NaC/CaO catalysts were prepared by varying the amount of sodium from 1–5 wt%. The aminolysis of used cottonseed oil was performed with diethanolamine (DEA/oil = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; m/m) at 110 °C in the presence of 5 wt% catalyst. FAA yield was found to increase as the amount of sodium ion in CaO were increased from 1 to 3 wt% (Fig. 4A). However, further increase in Na+ ion concentration does not have an influence on the reaction rate and hence, 3-NaC/CaO-450 catalyst was selected for optimizing the other parameters to attain the minimum time for the complete aminolysis of used cotton seed oil.
1; m/m) at 110 °C in the presence of 5 wt% catalyst. FAA yield was found to increase as the amount of sodium ion in CaO were increased from 1 to 3 wt% (Fig. 4A). However, further increase in Na+ ion concentration does not have an influence on the reaction rate and hence, 3-NaC/CaO-450 catalyst was selected for optimizing the other parameters to attain the minimum time for the complete aminolysis of used cotton seed oil.
A series of aminolysis of used cottonseed oil with DEA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, m/m) at 110 °C were performed in the presence of 3-NaC/CaO-450 by varying its amount from 1 to 10 wt% (catalyst/oil) to find the optimal catalyst concentration. The reaction rate of aminolysis (FAA yield) increases as catalyst concentration was increased from 1 to 5 wt%. A further increase in catalyst concentration does not alter the reaction rate significantly and hence, the aminolysis reactions were performed with a 5 wt% catalyst concentration for optimization the other parameters (Fig. 4B).
6, m/m) at 110 °C were performed in the presence of 3-NaC/CaO-450 by varying its amount from 1 to 10 wt% (catalyst/oil) to find the optimal catalyst concentration. The reaction rate of aminolysis (FAA yield) increases as catalyst concentration was increased from 1 to 5 wt%. A further increase in catalyst concentration does not alter the reaction rate significantly and hence, the aminolysis reactions were performed with a 5 wt% catalyst concentration for optimization the other parameters (Fig. 4B).
In order to find the optimum temperature, a series of aminolysis reactions were conducted in the presence of 5 wt%, (catalyst/used cottonseed oil) 3-NaC/CaO-450 catalyst from 30 °C to 130 °C. The fatty acid amide conversion increases as temperature of the reaction was increased to 110 °C as shown in Fig. 4C. Further increase in reaction temperature does not affect the FAA yield significantly and hence, all aminolysis reactions have been carried out at 110 °C. Also, the complete aminolysis (98 ± 2% m/m) of used cotton seed oil has been possible at room temperature (35 °C) though needed longer reaction duration (∼2 h).
The optimum DEA/UCSO molar ratio has been determined by performing the reactions with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8
1 to 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios at 110 °C using 5 wt% of NaC/CaO catalyst. The rate of formation of FAA increases as diethanolamine/oil molar ratio was increased from 3
1 molar ratios at 110 °C using 5 wt% of NaC/CaO catalyst. The rate of formation of FAA increases as diethanolamine/oil molar ratio was increased from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4D). Further increase in diethanolamine/oil molar ratio does not influence the rate of aminolysis significantly.
1 (Fig. 4D). Further increase in diethanolamine/oil molar ratio does not influence the rate of aminolysis significantly.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was required though the reaction time was same for the complete aminolysis of all feedstocks (Table 3).
1) was required though the reaction time was same for the complete aminolysis of all feedstocks (Table 3).
| S. No. | Type of feedstock | DEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feedstock molar ratio feedstock molar ratio |
|---|---|---|
| 1 | CSO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | SO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | UCSO | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | MF | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | FAMEs (UCSO) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | FAMEs (MF) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | ML | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The catalyst reusability study shows the steady loss of the catalytic activity which could be due to the partial leaching of the active species from the catalyst support. In order to reckon the homogeneous contribution involved in the catalyst activity, the catalyst, 3-NaC/CaO-450 catalyst (500 mg, equivalent to 5 wt% of oil used later for the aminolysis), has been refluxed with diethanolamine for 0.5 h at 110 °C and then catalyst was separated from diethanolamine by filtration. Diethanolamine thus obtained was mixed with used cotton seed oil to maintain amine/oil molar ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and reaction mixture was stirred at 110 °C for 0.5 h. Under these experimental conditions no conversion of oil to FAA has been found. This experiment clearly ruled out the possibility of aminolysis due to any leached species, and also supports that solid catalyst is accountable for the entire catalytic activity.
1, and reaction mixture was stirred at 110 °C for 0.5 h. Under these experimental conditions no conversion of oil to FAA has been found. This experiment clearly ruled out the possibility of aminolysis due to any leached species, and also supports that solid catalyst is accountable for the entire catalytic activity.
Furthermore, the loss of catalytic activity was examined by ICP (Inductively Coupled Plasma Spectrometry), powder XRD, FTIR and TEM analysis of fresh and recycled NaC/CaO. The ICP analysis suggested leaching of Ca metal ions but in negligible low amount (less than 5 ppm) whereas there was no measureable loss of Na metal ions. The FTIR analysis shows no change in the fresh and recycled NaC/Cao (Fig. S6B, ESI†). The powder XRD analysis of fresh and recycled catalyst shows the presence of new unknown phase peaks which suggested the distortion of structure after reaction (Fig. S6A, ESI†). On the other hand TEM analysis shows the aggregation of particles which could be due the multiple reuse of NaC/CaO (Fig. S7, ESI†). On the basis of all these studies, the distortion of structure of NaC/CaO (hexagonal phase of CaO) could be the most possible reason for the loss of catalytic activity for aminolysis.
3.3 Alcoholysis reaction
In order to show the efficacy of NaC/CaO for the reaction other than aminolysis, a series of reactions have been performed for the complete alcoholysis (>98% yield) of variety of triglycerides.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) at 65 °C were carried out in the presence of nanocrystalline 3-NaC/CaO-450 by varying its concentration from 1 to 10 wt% (catalyst/oil) in order to find the optimum catalyst concentration. The yield of FAMEs was found maximum (98 ± 2%) as catalyst concentration was increased from 1 to 5 wt%. Further increase in catalyst concentration does not have an effect on yield of FAMEs significantly (Fig. 6A). Other parameters of the transesterification reactions were further optimized with catalyst concentration of 5 wt% (catalyst/oil).
1 molar ratio) at 65 °C were carried out in the presence of nanocrystalline 3-NaC/CaO-450 by varying its concentration from 1 to 10 wt% (catalyst/oil) in order to find the optimum catalyst concentration. The yield of FAMEs was found maximum (98 ± 2%) as catalyst concentration was increased from 1 to 5 wt%. Further increase in catalyst concentration does not have an effect on yield of FAMEs significantly (Fig. 6A). Other parameters of the transesterification reactions were further optimized with catalyst concentration of 5 wt% (catalyst/oil).
The prepared catalyst was used for a series of transesterification reactions of UCSO by varying the reaction temperature from 35 to 75 °C. The FAMEs yield was reached to 98% as temperature of the reaction was increased from 35 °C to 65 °C. Further increase in reaction temperature does not affect the activity of catalyst (Fig. 6B) and hence, further all transesterification reactions have been performed at 65 °C.
The effect of methanol/oil molar ratio on transesterification reaction is one of the most important parameter which affects the FAMEs yield as well as cost of the production. Minimum 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of methanol to oil required for the complete conversion of vegetable oil to FAMEs. However, transesterification being a reversible reaction, usually carried out with high amount of methanol to shifts the equilibrium in forward direction to achieve the maximum FAMEs yield in short reaction time. To find out the optimum methanol amount in present study, the transesterification reactions of UCSO were performed with 3
1 molar ratio of methanol to oil required for the complete conversion of vegetable oil to FAMEs. However, transesterification being a reversible reaction, usually carried out with high amount of methanol to shifts the equilibrium in forward direction to achieve the maximum FAMEs yield in short reaction time. To find out the optimum methanol amount in present study, the transesterification reactions of UCSO were performed with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 18
1 to 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios (methanol/oil) at 65 °C using 5 wt% of 3-NaC/CaO-450 solid catalyst. The complete transesterification (98 ± 2% yield) was observed as methanol/oil molar ratio was increased from 3
1 molar ratios (methanol/oil) at 65 °C using 5 wt% of 3-NaC/CaO-450 solid catalyst. The complete transesterification (98 ± 2% yield) was observed as methanol/oil molar ratio was increased from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 12
1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 6C). The transesterification reactions for optimization the other parameters were further studied with 12
1 (Fig. 6C). The transesterification reactions for optimization the other parameters were further studied with 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of methanol/oil molar ratio.
1 of methanol/oil molar ratio.
Presence of water in feedstock always inherited the reaction progress and more than 0.3 wt% leads to the saponification instead of transesterification in the presence of homogenous catalyst.12 UCSO used in present study were found to have 0.4 wt% moisture contents and transesterification reaction of the same using homogeneous catalyst (NaOH or KOH) leads to the soap formation. However, complete conversion was observed when same reaction catalyzed by nanocrystalline 3-NaC/CaO-450. In order to determine the moisture resistance of the 3-NaC/CaO-450, the transesterification reactions of UCSO were performed in the presence of 0.4–10.4 wt% (water/oil) water. The solid catalyst was found to be effective for the complete transesterification of UCSO in 5.5 h even in the presence of 10.4 wt% of moisture contents (Fig. 6D). Addition of higher water content (12.4 wt%) decreases the FAMEs yield (61%) and further increase leads to the soap formation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol to oil molar ratio in presence of 5 wt% catalyst at 65 °C has been employed. The catalyst was found to be effective for the complete alcoholysis and yielded 99% respected fatty acid alkyl ester (Fig. 7A). The TOF was found to be 39.6, 23.8, 15.8 and 11.3 h−1 for methanol, ethanol, propanol and butanol, respectively. The higher reaction duration of 50 min, 75 min and 105 min with ethanol, propanol and butanol, respectively, in comparison to methanolysis reaction (30 min) suggests that the inherent carbon chain length of the alcohols influenced catalyst activity. The influence on NaC/CaO activity with increasing carbon chain length could be attributed to the reduction in acidic strength of alcohols and change in alcohol solubility.
1 ethanol to oil molar ratio in presence of 5 wt% catalyst at 65 °C has been employed. The catalyst was found to be effective for the complete alcoholysis and yielded 99% respected fatty acid alkyl ester (Fig. 7A). The TOF was found to be 39.6, 23.8, 15.8 and 11.3 h−1 for methanol, ethanol, propanol and butanol, respectively. The higher reaction duration of 50 min, 75 min and 105 min with ethanol, propanol and butanol, respectively, in comparison to methanolysis reaction (30 min) suggests that the inherent carbon chain length of the alcohols influenced catalyst activity. The influence on NaC/CaO activity with increasing carbon chain length could be attributed to the reduction in acidic strength of alcohols and change in alcohol solubility.
Presence of FFAs in feedstock is also critical like moisture content and leads to saponification if FFAs is more than 0.5 wt% (in case of homogeneous catalyst).27 UCSO used in present study has 2.2 wt% FFAs and transesterification reaction of the same using NaOH or KOH as homogenous catalyst leads to the saponification instead of transesterification. However, 3-NaC/CaO-450 was found efficient and yielded the complete conversion of oil to FAMEs in 0.5 h. The optimal FFA tolerance of the prepared catalyst towards the transesterification reactions was observed using variety of natural feedstocks having different amount of FFAs (wt%) like, animal fat (1.3), virgin soybean oil (0.2), cotton seed oil (0.11), used cotton seed oil (2.2), castor oil (2.0), karanja oil (4.1) and jatropha oil (8.4). The solid catalyst was found effective for the complete transesterification of all the feedstocks used (having upto 8.4 wt% FFAs) as shown in Fig. 7B. However, high FFAs affect the catalytic activity as reaction time increases with increase in FFAs content (karanja and jatropha oil).
4. Conclusions
Solid catalyst Na2CO3 impregnated CaO was prepared in nanoparticles form (particle size = 25 nm) by very simple method and used for solvent free one step aminolysis and transesterification reactions of a variety of feedstocks. NaC/CaO was found to complete the aminolysis and alcoholysis of UCSO using diethanolamine and methanol, respectively, in 0.5 h at 110 °C and 65 °C, respectively. The catalyst was found to be equally efficient for the aminolysis and alcoholysis of other feedstocks. The turnover frequency was found to be 39.6 h−1 for both the aminolysis and alcoholysis reactions of used cotton seed oil using NaC/CaO. The catalyst was efficient even in the presence of moisture content up to 10.4% and also completes the transesterification of UCSO with long chain (1–4 carbon) alcohols. The catalyst shows excellent reusability and used for six successive catalytic cycles for the aminolysis of UCSO.Acknowledgements
We are thankful to Center for University-Wide Research Facilities, Chonbuk National University, Jeonju, South Korea for powder XRD, FE-SEM, TEM and DLS analysis.Notes and references
- B. Subhasree, R. Baskar, R. Laxmi Keerthana, R. Lijina Susan and P. Rajasekaran, Food Chem., 2009, 115, 1213–1220 CrossRef CAS.
- R. Luque, L. Herrero-Davila, J. M. Campelo, J. H. Clark, J. M. Hidalgo, D. Luna, J. M. Marinas and A. A. Romero, Energy Environ. Sci., 2008, 1, 542–564 CAS.
- U. Biermann, W. Friedt, S. Lang, W. Lühs, G. Machmüller, U. O. Metzger, M. R. Gen Klaas, H. J. Schäfer and M. P. Schneider, in Biorefineries-Industrial Processes and Products, Wiley-VCH Verlag GmbH, 2008, pp. 253–289, DOI:10.1002/9783527619849.ch25.
- T. M. Kuo, H. Kim and C. T. Hou, Curr. Microbiol., 2001, 43, 198–203 CrossRef CAS PubMed.
- H. Maag, J. Am. Oil Chem. Soc., 1984, 61, 259–267 CrossRef CAS.
- K. J. Liu, A. Nag and J.-F. Shaw, J. Agric. Food Chem., 2001, 49, 5761–5764 CrossRef CAS PubMed.
- R. Alcantara, J. Amores, L. Canoira, E. Fidalgo, M. J. Franco and A. Navarro, Biomass Bioenergy, 2000, 18, 515–527 CrossRef CAS.
- S. Stournas, E. Lois and A. Serdari, J. Am. Oil Chem. Soc., 1995, 72, 433–437 CrossRef CAS.
- J. M. Encinar, J. F. González, J. J. Rodríguez and A. Tejedor, Energy Fuels, 2002, 16, 443–450 CrossRef CAS.
- U. Rashid, F. Anwar, B. R. Moser and S. Ashraf, Biomass Bioenergy, 2008, 32, 1202–1205 CrossRef CAS.
- P. Sun, J. Sun, J. Yao, L. Zhang and N. Xu, Chem. Eng. J., 2010, 162, 364–370 CrossRef CAS.
- D. Kumar and A. Ali, Energy Fuels, 2013, 27, 3758–3768 CrossRef CAS.
- R. S. Watkins, A. F. Lee and K. Wilson, Green Chem., 2004, 6, 335–340 RSC.
- M. C. de Zoete, A. C. Kock-van Dalen, F. van Rantwijk and R. A. Sheldon, J. Mol. Catal. B: Enzym., 1996, 1, 109–113 CrossRef CAS.
- N. P. Awasthi and R. P. Singh, Eur. J. Lipid Sci. Technol., 2009, 111, 202–206 CrossRef CAS.
- S. H. Feairheller, R. G. Bistline Jr, A. Bilyk, R. L. Dudley, M. F. Kozempel and M. J. Haas, J. Am. Oil Chem. Soc., 1994, 71, 863–866 CrossRef CAS.
- W. E. Levinson, T. M. Kuo and C. P. Kurtzman, Enzyme Microb. Technol., 2005, 37, 126–130 CrossRef CAS.
- M. J. J. Litjens, M. Sha, A. J. J. Straathof, J. A. Jongejan and J. J. Heijnen, Biotechnol. Bioeng., 1999, 65, 347–356 CrossRef CAS PubMed.
- J. Rawlins, M. Pramanik and S. Mendon, J. Am. Oil Chem. Soc., 2008, 85, 783–789 CrossRef CAS.
- B. K. Sharma, A. Adhvaryu and S. Z. Erhan, J. Agric. Food Chem., 2006, 54, 9866–9872 CrossRef CAS PubMed.
- E. A. J. Al-Mulla, W. M. Z. W. Yunus, N. A. B. Ibrahim and M. Z. A. Rahman, J. Oleo Sci., 2009, 58, 467–471 CrossRef CAS PubMed.
- A. Bilyk, R. Bistline Jr, G. Piazza, S. Feairheller and M. Haas, J. Am. Oil Chem. Soc., 1992, 69, 488–491 CrossRef CAS.
- E. Jordan Jr and W. Port, J. Am. Oil Chem. Soc., 1961, 38, 600–605 CrossRef.
- A. L. McKenna and W. C. C. H. C. Division, Fatty amides: synthesis, properties, reactions, and applications, Witco Chemical Corporation, Humko Chemical Division, 1982 Search PubMed.
- R. Bistline, J. Hampson and W. LinField, J. Am. Oil Chem. Soc., 1983, 60, 823–828 CrossRef CAS.
- R. G. Bistline, E. W. Maurer, F. D. Smith and W. M. Linfield, J. Am. Oil Chem. Soc., 1980, 57, 98–103 CrossRef CAS.
- E. Lotero, Y. Liu, D. E. Lopez, K. Suwannakarn, D. A. Bruce and J. G. Goodwin, Ind. Eng. Chem. Res., 2005, 44, 5353–5363 CrossRef CAS.
- C. R. Venkat Reddy, R. Oshel and J. G. Verkade, Energy Fuels, 2006, 20, 1310–1314 CrossRef.
- A. Demirbas, Prog. Energy Combust. Sci., 2007, 33, 1–18 CrossRef CAS.
- D. Kumar and A. Ali, Energy Sources, Part A, 2014, 36, 1093–1102 CrossRef CAS.
- N. Degirmenbasi, N. Boz and D. M. Kalyon, Appl. Catal., B, 2014, 150–151, 147–156 CrossRef CAS.
- G. V. Smith and F. Notheisz, in Heterogeneous Catalysis in Organic Chemistry, ed. G. V. S. Notheisz, Academic Press, San Diego, 1999, pp. 1–28, DOI:10.1016/b978-012651645-6/50001-9.
- S. B. Qadri, E. F. Skelton, D. Hsu, A. D. Dinsmore, J. Yang, H. F. Gray and B. R. Ratna, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 60, 9191–9193 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, spectrum (XRD, FT-IR, mass and 1H-NMR), Fig. S1–S7. See DOI: 10.1039/c6ra13446g |
| This journal is © The Royal Society of Chemistry 2016 |