Simply controllable growth of single crystal plasmonic Au–Ag nano-spines with anisotropic multiple sites for highly sensitive and uniform surface-enhanced Raman scattering sensing†
Abstract
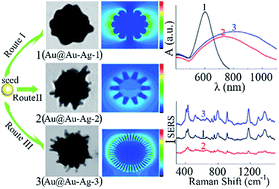
The design and simple synthesis of plasmonic noble metal nanostructures with a highly tunable local surface plasmon resonance (LSPR) band and high density of “hot spots” is pivotal for multifarious wavelength matched, sensitive surface-enhanced Raman scattering (SERS) detection. Herein, inspired by the function of silver ions in nanorod aspect ratio control, a series of Au core@Au–Ag alloy spine nanostructures (Au@Au–Ag) with a controllable surface morphology and size were synthesized via a simple and cheap AgNO3-controlled chemistry and seed-mediated method. In addition, multifunctional L-DOPA was employed, which acts as a reducing agent and capping agent to reduce the Au/Ag ions, stabilize the nanostructures and direct a protuberant growth, so synthetic steps were simplified. Growth of anisotropic multiple sites form a high density of nano-spines, which form dense “hot spots” and a tunable LSPR band from visible to near-infrared wavelength. The correlations between the excitation laser wavelength and electromagnetic (EM) field distribution and enhancement in the Au@Au–Ag were further investigated. The strongest EM field was generated in the gap or tip area of the nanostructures and producing a compact nano-spine is necessary to generate a high density of “hot spots” and obtain a near-infrared signal. The calculated SERS-enhancement factor value of the Au@Au–Ag substrate is higher than 109. Finally, the highly controllable nanoantenna structures make the Au@Au–Ag be a multifarious wavelength compatible, sensitive SERS substrate for practical sensing applications. We further employ the surface-enhanced resonance Raman scattering detection of malachite green using Au@Au–Ag as a substrate under the interference of various ions and organics, which shows excellent anti-interference performance and reproducibility, the relative standard deviation is only 8.2%. Moreover, under the interference of various impurities in complex environmental water samples, the Au@Au–Ag substrate shows high sensitivity with a 100 pM limit of detection.

 Please wait while we load your content...
Please wait while we load your content...