Polyphenolic profiles and the in vivo antioxidant effect of nipa vinegar on paracetamol induced liver damage†
Boon Kee Behab,
Nurul Elyani Mohamadc,
Swee Keong Yeapa,
Kian Lam Limd,
Wan Yong Hoe,
Hamidah Mohd Yusofa,
Shaiful Adzni Sharifuddinb,
Anisah Jamaluddinb,
Kamariah Long*b and
Noorjahan Banu Alitheenac
aInstitute of Bioscience, Universiti Putra Malaysia, Serdang, Selangor, Malaysia
bBiotechnology Research Centre, Malaysian Agricultural Research and Development Institute (MARDI), Serdang, Selangor 43400, Malaysia. E-mail: amai@mardi.gov.my; Tel: +60-3-89537238
cDepartment of Cell and Molecular Biology, Faculty of Biotechnology and Biomolecular Science, Universiti Putra Malaysia, Serdang, Selangor 43400, Malaysia
dFaculty of Medicine and Health Sciences, Universiti Tunku Abdul Rahman, Sungai Long Campus, Jalan Sungai Long, Bandar Sungai Long, Cheras, Kajang 43000, Selangor, Malaysia
eSchool of Biomedical Sciences, The University of Nottingham Malaysia Campus, Jalan Broga, 43500 Semenyih, Selangor, Malaysia
First published on 17th June 2016
Abstract
Plant-based vinegar is proclaimed to have multiple health benefits due to the presence of polyphenol. However, not all vinegars have similar antioxidant activities. Nipa (Nypa fruticans) vinegar is one type of vinegar that has been widely consumed in Philippines and Malaysia. In this study, the antioxidant activity, polyphenolic acid profiles and antioxidant benefits to revert paracetamol-induced liver damage in mice in vivo have been evaluated. Nipa vinegar was found to contain antioxidant activity attributed to the presence of gallic acid, protocatechuic acid and 4-hydroxybenzoic acid. Continuous consumption of nipa vinegar for 14 days was able to recover the liver damage induced by paracetamol in a dosage dependent manner as indicated by the liver H&E histopathology, recovery serum liver profile (AST, ALT and ALP) and suppression of liver cytochrome P450 2E1 expression. These effects contributed to the antioxidant and anti-inflammatory effects of nipa vinegar. Similar to other types of vinegars, nipa vinegar, which is rich in polyphenolic acids, can contribute to in vivo anti-oxidation, anti-inflammation and liver protection effects in paracetamol treated mice.
1. Introduction
Paracetamol (acetaminophen) is one of the top 10 causative agents which contribute to drug-induced liver damage, found especially in developed nations such as Australia, European regions and the United States. Wide availability and easy accessibility of this analgesic agent has contributed to its popularity as one of the most commonly used pain killers, which subsequently contributed to intentional or unintentional overdoses.1 Over consumption of paracetamol will activate hepatic cytochrome P450 enzyme, metabolizing them into a toxic alkylated metabolite known as N-acetyl-p-benzoquinone imine (NAPQI), which will then deplete the liver antioxidant peptide glutathione (GSH) during the detoxification process.2 As a result, maintaining adequate cellular antioxidants especially the GSH level has been proposed as one strategy to prevent and treat drug-induced liver diseases.3 To achieve this, food scientists have suggested the use of dietary ingredients or functional food to enhance the body’s antioxidant level.4Among the dietary and functional food ingredients, vinegar, which is widely recognised as acidic seasoning, has received great attention recently due to its proclaimed beneficial effects. Vinegar has been recorded as a source of polyphenolic and organic acids.5 However, Nishidai et al.6 have found that not all the vinegar contained equal levels of antioxidant. For example, synthetic vinegar did not contain antioxidants but surprisingly enhanced the in vivo antioxidant effect in paracetamol-induced liver damaged mice.7 On the other hand, pineapple vinegar7 and black vinegar were recorded to have high antioxidant effects both in vitro and in vivo.4 Thus, it is necessary to evaluate and compare the antioxidant value and benefits of different sources of vinegars.
Nipa sap or more commonly known as “air nira” in Malaysia is a type of sweet juice collected from the flower cluster of nipa palm (Nypa fruticans). Although fresh nipa sap does not contain any alcohols or acids, its highly fermentable nature has favoured the processing of this sap into alcoholic beverages (name as tuba/soom in Philippines, tuak in Indonesia or toddy in Malaysia)8 or vinegar.9 The plantation of nipa palm is increasing due to its very high yields of sugar-rich sap as compared to other crops with lower maintenance cost.10 Many studies have reported the antioxidant,9,11 anti-infective12–14 effects of fruits and leaves of nipa palm. Nipa vinegar has been commonly used in cooking and as traditional medicine in treating diabetes, high blood pressure and gout.15 However, the bioactivities and efficacies of this widely consumed nipa vinegar are still not well studied. Thus, the purpose of this study was to evaluate the polyphenolic acid content of nipa vinegar. Furthermore, the regulation of liver oxidation, cytochrome P450 enzymes and the inflammatory mechanisms by nipa vinegar, which contributed to the healing of paracetamol-induced liver damage, were evaluated.
2. Materials and methods
2.1. Preparation of nipa vinegar
Fresh nipa sap was obtained from a local market (Pasar Borong Selangor, Selangor, Malaysia). The juice was then filtered using a muslin cloth to remove the solid debris sediment. After that, the filtrate was pasteurized at 90 °C for 15 minutes and allowed to fast cool to 30 °C. The pasteurized nipa sap was then inoculated with 0.2 g L−1 of Saccharomyces cerevisiae 7013 INRA (DSM, Netherlands) with an OD620 of ∼ 0.5. Yeast broth fermentation was carried out at 30 ± 1 °C for 10 days. The alcoholic nipa broth was then further inoculated with 20% v/v Acetobacter aceti var Europeans (Culture collection center of Malaysian Agriculture and Research Development Institute, Malaysia) with an OD620 of ∼ 0.5 at 30 ± 1 °C for 1 month. The fermented nipa vinegar with the final titratable acidity of 6% v/v was kept in an air-tight storage tank for 1 month for the maturation process. Finally, the nipa vinegar was filtered and stored at 4 °C prior to the following assays. Synthetic vinegar (Yakin, Malaysia) was purchased from the local store (Tesco, Malaysia). Both nipa and synthetic vinegar were diluted to 4% v/v acidity prior to be used.2.2. In vitro antioxidant assay and HPLC polyphenolic acid quantification
The ferric reducing ability plasma (FRAP) assay and free phenolic acids of nipa sap, alcohol and vinegar were determined according to previous studies.7 FRAP assays were done using iron(II) sulfate (FeSO4) as a standard and the final result was expressed in μM of iron(II) (Fe2+). Samples of 20 μL of vinegar, 150 μL of the FRAP working solution (4 mL tripyridyl triazine {TPTZ} and 4 mL of iron(III) chloride hexahydrate {FeCl3·6H2O} in 40 mL acetate buffer) were mixed and incubated for 10 minutes. The final absorbance was measured at 593 nm using an enzyme-linked immunosorbent assay (ELISA) plate reader (Bio-tek Instrument, USA).Soluble phenolic acids, which are present in fresh nipa sap, alcoholic nipa broth and nipa vinegar, were quantified using high-performance liquid chromatography (HPLC) Alliance Separation Module (Waters 2695) equipped with a Diode Array detector (Waters 2996) at the wavelength of 270 nm. A reverse phase C18 analytical column (150 mm × 4.6 mm × 3.5 μm, XBridge C18, Waters, USA) was utilized with the oven temperature set at 25 °C. An aliquot of 10 μL of each sample were injected using an auto-sampler. The mobile phase used for separation was 0.1% formic acid (mobile phase A) and methanol (mobile phase B) under gradient mode. The flow rate was set at 0.7 mL min−1. Quantification was made using the external standard method. The total soluble phenolic acid content was calculated based on the sum of gallic acid, protocatechuic acid (3,4-dihydroxybenzoic acid), and 4-hydroxybenzoic acid.
2.3. Experimental animals and design
Male Balb/c mice obtained from Faculty of Veterinary Sciences, Universiti Putra Malaysia (UPM) (aged 4–5 weeks old with average body weight of 20–22 g) (n = 42) were acclimatized for 1 week in an animal house (23 ± 2 °C) with a 12 hour daily lighting schedule. The mice were fed a standard pellet diet and distilled water ad libitum. This study was conducted according to the standard ethical guidelines & was approved by the Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia (UPM/FPV/PS/3.2.1.551/AUP-R168).After acclimatization, the mice were randomly assigned into 7 groups with six mice per group (n = 6). Group 1 (N) was normal healthy control and received saline. Group 2 to 7 were orally administrated with 250 mg per kg body weight per day of paracetamol for 7 days. After that, mice from each group were subjected to the following treatment for 14 days:
Group 1 (N): normal control group without paracetamol induction receiving distilled water only.
Group 2 (UT): untreated paracetamol control group receiving distilled water only.
Group 3 (S): positive control group receiving 50 mg kg−1 BW of silybin.
Group 4 (SH): acetic acid control group receiving 2 mL kg−1 BW of synthetic vinegar.
Group 5 (SL): acetic acid control group receiving 0.08 mL kg−1 BW of synthetic vinegar.
Group 6 (NH): treatment group receiving 2 mL kg−1 BW of nipa vinegar.
Group 7 (NL): treatment group receiving 0.08 mL kg−1 BW of nipa vinegar.
2.3.5.1. FRAP assay. 80 μL of the liver homogenate and 150 μL of the master solution (30 mL of 300 mM acetate buffer to 3 mL of 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution and 3 mL of 20 mM FeCl3·6H2O solution in 40 mM HCl) were mixed and incubated for 10 minutes. The absorbance was measured using an ELISA plate reader (Bio-tek Instrument, USA) at 593 nm.
2.3.5.2. SOD assay. The liver homogenate and master solution (0.1 mol L−1 phosphate buffer, 0.15 mg mL−1 sodium cyanide in 0.1 mol L−1 ethylenediaminetetraacetic acid (EDTA), 1.5 mmol L−1 nitroblue tetrazolium (NBT) and 0.12 mmol L−1 riboflavin) were mixed and the absorbance was measured using an ELISA plate reader (Bio-tek Instrument, USA) at 560 nm.
2.3.5.3. MDA assay. 200 μL of the liver homogenate, 800 μL of PBS, 25 μL 8.8 mg mL−1 butylhydroxytoluene (BHT) and 500 μL of 50% trichloroacetic acid (TCA) were mixed, vortexed and incubated on ice for 2 hours. Next, the mixture was spun at 2000 g for 15 minutes. Then, 1 mL of the supernatant, 75 μL of 0.1 M EDTA and 250 μL of 0.05 M 2-thiobarbituric acid (TBA) were mixed in new tubes and boiled for 15 minutes. The absorbance was measured using an ELISA Reader (Bio-tek Instrument, USA) at 532 and 600 nm.
2.3.5.4. NO assay. 50 μL of the liver homogenate, 130 μL of distilled water and 20 μL of the Griess reagent were mixed in a 96 well plate and incubated for 30 minutes at room temperature. The absorbance was measured using an ELISA Reader (Bio-tek Instrument, USA) at 540 nm.
2.3.5.5. Reduced glutathione (GSH) activity. 10 mL of the liver homogenate and 150 μL of working solution (1.5 mg mL−1 DTNB solution, 6 units per mL glutathione reductase and 1× assay buffer) were mixed in a 96 well plate and incubated for 5 minutes. Then, 50 mL of nicotinamide adenine dinucleotide phosphate (NADPH) solution (0.16 mg mL−1) was added to the plate. The absorbance was measured using ELISA plate reader (Bio-tek Instrument, USA) at 412 nm at 1 minute intervals for 5 minutes.
2.4. Statistical analyses
All experiments were done in triplicate. The means ± SD were calculated for each group using one-way analysis of variance (ANOVA) and Duncan’s multiple range test by SPSS 16 software. The significance was set at P < 0.05 against the untreated group.3. Results
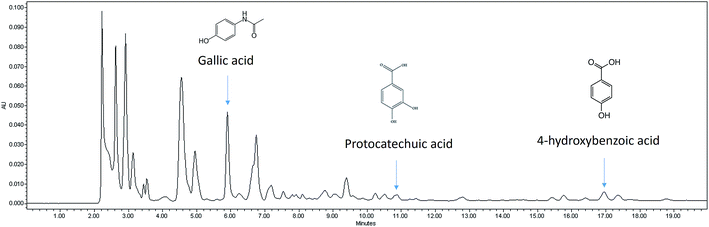
3.1. In vitro polyphenolic acid content and antioxidant effect of nipa vinegar
The FRAP assay was carried out to determine the antioxidant capacity. In addition, selected polyphenolic acid content that contributed to the antioxidant effect was also evaluated using HPLC-PDA and the representative HPLC chromatogram for nipa vinegar is shown in Fig. 1. Nipa vinegar was recorded with the highest antioxidant capacity (176 μg TE per mL) compared to fresh nipa sap and nipa alcohol (Table 1). Higher antioxidant capacity in nipa vinegar may be due to the presence of higher phenolic acids, particularly gallic acid (143 μg mL−1), protocatechuic acid (61 μg mL−1), and 4-hydroxybenzoic (52 μg mL−1). In terms of fresh nipa sap and nipa alcohol, only gallic acid at a much lower concentration was detected (Table 1).| Synthetic vinegar | Fresh nipa sap | Nipa alcohol | Nipa vinegar | |
|---|---|---|---|---|
| FRAP (μg TE mL−1) | — | 28 | 55 | 176 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Phenolic acid derivatives (HPLC) | ||||
| Gallic acid (μg mL−1) | — | 4 ± 1 | 22 ± 1 | 143 ± 2 |
| Protocatechuic acid (μg mL−1) | — | — | — | 61 ± 4 |
| 4-Hydroxybenzoic (μg mL−1) | — | — | — | 52 ± 2 |
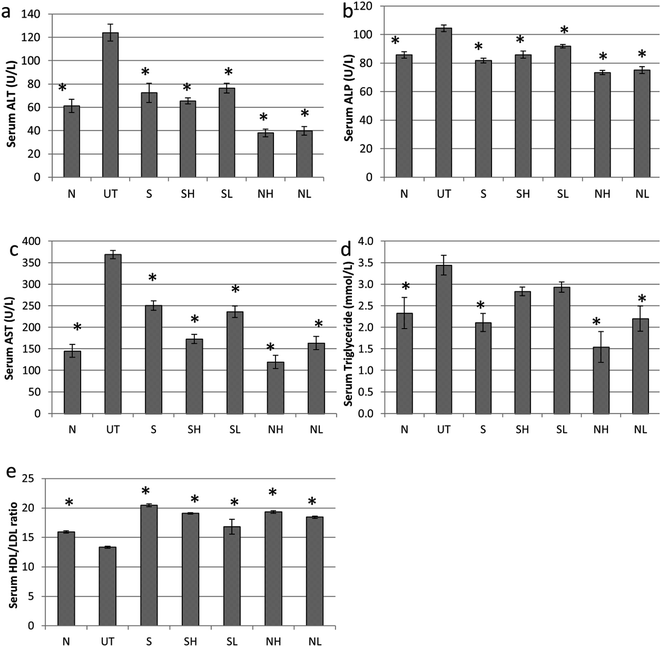
3.2. Nipa vinegar attenuates paracetamol induced hepatotoxicity in mice
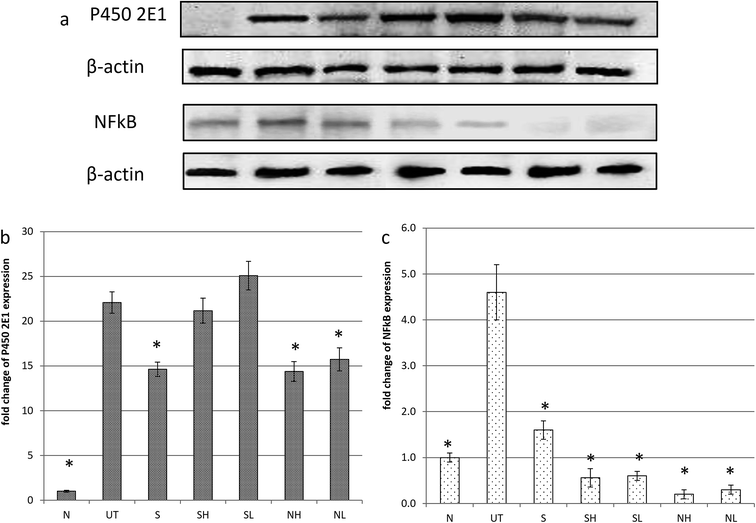
Paracetamol has induced hepatotoxicity in untreated mice with indications such as elevation of serum liver markers (ALT, ALP and AST) and triglyceride (TG) level associated with a lower high-density lipoprotein vs. low-density lipoprotein (HDL/LDL) ratio (Fig. 2). In addition, pyknotic nuclei (black rectangle), ballooned hepatocytes (green circle) and sinusoidal congestion (SC) (Fig. 3b) was observed in the liver histopathology of the untreated mice group. On the other hand, groups treated with nipa vinegar were recorded to have a reduction of the serum liver enzyme profile and TG level with a higher ratio of HDL/LDL in a dosage dependent manner (Fig. 2). It was also clearly depicted in the histological analysis, where extensive reductions of pyknotic nuclei, ballooned hepatocytes and sinusoidal congestion associated with a higher incidence of binuclear hepatocytes were recorded in the nipa vinegar treated groups (Fig. 3f and g). Both silymarin and acetic acid controls were able to reduce the serum liver enzyme markers (Fig. 2). Similar to the high dosage of nipa vinegar, binuclear hepatocytes with a lower incidence of sinusoidal congestion and ballooned hepatocytes were observed in the liver histopathology of silymarin treated mice indicating the recovery of the damaged liver by the treatment (Fig. 3c).3.3. Nipa vinegar suppresses cytochrome P450 activity and inflammation in paracetamol induced hepatotoxic mice
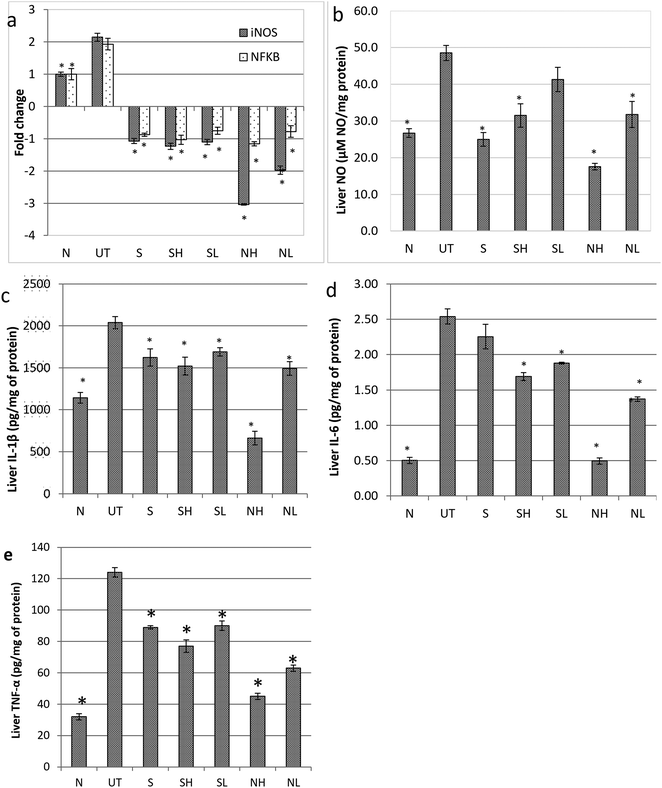
Untreated mice fed with paracetamol were observed with a drastic elevation of cytochrome P450 2E1 (CYP2E1) enzyme level as compared to the healthy normal control mice. Consistent with the serum biochemical profile, nipa vinegar and the positive control silymarin were able to down regulate the expression of the CYP2E1 enzyme significantly (p < 0.05). On the other hand, both concentrations of acetic acid failed to suppress the expression of the CYP2E1 enzyme (Fig. 4).To evaluate the impact of nipa vinegar on altering the effects of paracetamol induced liver inflammation, mRNA expression of inflammatory markers such as iNOS and NFkB in the liver tissues was evaluated by qRT-PCR and protein expression of NFkB was further validated by western blot analysis. In addition, the nitric oxide level present in the liver sample was also measured. A two fold upregulation of iNOS and NFkB expression was observed in the untreated control mice as compared to the healthy control in both qPCR and western blot (only for NFkB) analyses. All the treated groups were recorded to downregulate both iNOS and NFkB expression. Among the treated groups, the most significant downregulation of iNOS was observed in the high concentration nipa vinegar treated mice (Fig. 5a). In terms of liver nitric oxide and proinflammatory cytokine IL-1β, IL-6 and TNF-α levels, untreated mice were found to have an elevated nitric oxide and proinflammatory cytokines level as compared to the healthy control mice. Conversely, similar with the trend of iNOS and NFkB expression, nitric oxide and proinflammatory cytokine IL-1β, IL-6 and TNF-α levels in the liver of nipa vinegar, high concentration acetic acid and positive control silymarin treated mice were significantly (p < 0.05) suppressed (Fig. 5b–e).
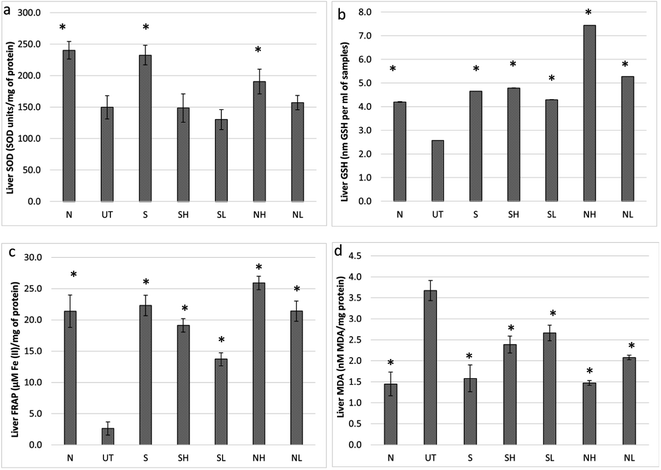
3.4. Nipa vinegar restores the antioxidant level in the liver of paracetamol intoxicated mice
Further insight on the regulation of CYP2E1 and proinflammatory markers by nipa vinegar was determined by comparing the antioxidant level in the liver tissues of controls and nipa vinegar treated mice. A significant (p < 0.05) decrease in the SOD (Fig. 6a), GSH (Fig. 6b) and FRAP (Fig. 6c) antioxidant capacity associated with an elevated level of malonaldehyde (MDA) (Fig. 6d) indicated the depletion of antioxidants and excessive oxidative stress in the liver obtained from mice intoxicated with paracetamol. On the other hand, nipa vinegar restored the SOD (Fig. 6a), GSH (Fig. 6b) and FRAP (Fig. 6c) antioxidant capacities and reduced the MDA level (Fig. 6d) in a dosage dependent manner. The acetic acid control was able to revert GSH (Fig. 6b) and FRAP (Fig. 6c) but not the SOD level (Fig. 6a), which subsequently brought down the MDA level (Fig. 6d) in the liver of the treated mice. The positive control silymarin had the best treatment effect in reversing the liver SOD back to the healthy control level (Fig. 6a).4. Discussion
Similar to black4 and pineapple vinegar,7 nipa vinegar also possessed antioxidant activity as indicated by FRAP assays. Gallic acid, protocatechuic acid, and 4-hydroxybenzoic acid were the phenolic acids that were detected (Fig. 1), which may contribute to the antioxidant activity of nipa vinegar (Table 1). Processing methods including fermentation have been recorded to have variable effects on the antioxidant activity of the plant materials.16 In this case, a 2 step fermentation of nipa was observed to lead to enhanced antioxidant capacity associated with a higher level of polyphenolic acids as compared to fresh nipa sap and nipa alcohol. Similarly, Roselle vinegar, fermented by yeast and acetobacter, was also reported to have enhanced total antioxidant activities and a higher level of the total phenolic content.16 Although the antioxidant capacity and the variety of phenolic acids detected in nipa vinegar were generally lower than pineapple vinegar, nipa sap is still a good raw material to produce vinegar due to its abundance and cheaper cost in Malaysia which make it more price competitive as compared to pineapple. Gallic acid, protocatechuic acid and 4-hydroxybenzoic acid are phenolic acids that are commonly found in edible plants.17,18 Gallic was reported as a better scavenger for deactivating a wide range of reactive oxygen species and reactive nitrogen species than the reference compound Trolox.17 In terms of protocatechuic acid and 4-hydroxybenzoic acid, the scavenging effect of these phenolic acids were found to depend on the hydrogen transfer from the hydroxyl group or single electron transfer from the di-anions of these phenolic acids. Thus, protocatechuic acid with an extra hydroxyl group was proposed as a better scavenger compared to 4-hydroxybenzoic acid.18 As these detected phenolic acids were previously reported as hepatoprotective agents,19–21 the effect of nipa vinegar in promoting the recovery of paracetamol induced liver damage was further evaluated.In this study, mice challenged with paracetamol were observed to have elevated serum lipid and liver profiles (Fig. 2). The increase of serum liver enzyme levels was attributed to the leakage of those enzymes from hepatocytes due to the increase in membrane permeability damage caused by impairment of the cytoplasm and mitochondria22 as indicated in the histopathological observation of the paracetamol treated mice (Fig. 3b). On the other hand, drastic elevation of the serum TG level and a decrease in the serum HDL/LDL ratio had indicated impaired fat metabolism and fatty changes due to the paracetamol induced liver toxicity.7 Nipa vinegar had promoted the recovery of paracetamol induced liver damage designated by the reduction of serum liver enzymes and TG levels. In addition, higher events of mitotic binuclear hepatocytes which were observed in the histopathological study of nipa vinegar treated mice also indicated the regeneration process by this vinegar in a dosage dependent manner (Fig. 3). This result was in accordance with the view that serum liver enzyme levels were restored when the hepatocytes were regenerated.23
CYP450 is the major liver enzyme responsible for metabolizing drugs such as paracetamol to the reactive toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI). Overexpression of CYP450, especially the isomer 2E1, was inducible by paracetamol or alcohol to promote hepatocyte apoptosis.24 This phenomenon was observed in the liver of the untreated mice. Conversely, nipa vinegar and positive control silybin had significantly (p < 0.05) reduced the level of CYP450 2E1 (Fig. 4). Previous studies have reported that antioxidants such as phenolic acids and flavonoids promote liver recovery from paracetamol damage via inhibiting the transport of excessive paracetamol through the hepatic organic anion-transporting polypeptide, thus lowering the level of P450 2E1 expression associated with less GSH depletion or generation of oxidative stress as this occurs in the liver.25,26 The bio-activation of paracetamol to NAPQI by the CYP450 enzyme had generated free radicals that deplete antioxidants, especially the peptide GSH level in the liver as NAPQI reacts fast with GSH, which led to an abnormal breakdown of lipid peroxidation that subsequently damaged the hepatocytes more significantly in the individual with low or depleted GSH levels.3,24 Potential of the toxic metabolite NAPQI to interact with GSH and DNA27 gives us a clue that enhancing or restoring the liver antioxidant level may help to promote recovery of paracetamol induced liver damage. In the untreated mice, liver antioxidants (indicated by levels of FRAP, SOD and GSH) were significantly downregulated while lipid peroxidation (indicated by the lipid peroxidation reactive end-product malondialdehyde) was significantly upregulated (Fig. 6). Certain food supplements such as N-acetyl cysteine, garlic and red wine have been recorded with the potential to counteract paracetamol toxicity.28 Unlike N-acetyl cysteine that reacts as a comparable scavenging agent as GSH to NAPQI,28 nipa vinegar, which contains phenolic acids, restored the liver antioxidant level and reduced lipid peroxidation in a dosage dependent manner. These results showed that nipa vinegar had promoted the recovery of paracetamol induced liver damage through stimulating the liver antioxidants, which subsequently prevent or reduce lipid peroxidation that damages hepatocytes.
In addition to the depletion of antioxidants, inflammation was also recorded in paracetamol induced liver damage. NF-kB is a stress induced-inflammatory related transcriptional factor. Elevation of NF-kB had promoted the expression of inflammatory genes including inducible nitric oxide synthase (iNOS)29 and proinflammatory cytokines such as IL-1β, IL-6 and TNF-α through recruitment of Kupffer cells and hepatic macrophages.29,30 Elevation of NF-kB, iNOS and these proinflammatory cytokines had promoted the generation of nitric oxide (NO) and reactive oxygen species, which react rapidly with superoxides generated by oxidative stress to produce peroxynitrite.30,31 However, the detoxification of this peroxynitrite, which is normally done by GSH, was depleted by the toxic by-product NAPQI originating from CYP450 activated paracetamol.32 Thus, the raise in iNOS and NO levels was always positively correlated with the elevation of serum liver enzyme levels7 while knocking-out of iNOS prevents 50% of the elevation of the serum ALT level of the paracetamol challenged iNOS knock-out mice.32 In this study, the level of NF-kB, iNOS and NO were significantly higher in the liver of the untreated mice whereas the nipa vinegar was able to reduce them in a dosage dependent manner. These results indicated that nipa vinegar can also control oxidative stress induced by paracetamol via downregulating of hepatic inflammation.
5. Conclusion
In short, nipa vinegar contains polyphenolic acids such as gallic acid and protocatechuic acid, which promote the recovery of paracetamol induced liver damage. This vinegar promotes the liver antioxidants and prevents inflammation to overcome lipid peroxidation caused by bioconversion of paracetamol to NAPQI by CYP450 2E1.Conflict of interest
There is no conflict of interest.Acknowledgements
This project was funded by a grant from Pembangunan RMK10, MARDI, Malaysia. Authors would like to thank Prof. Tan Soon Guan for proofreading and commenting on the manuscript.References
- D. Gunnell, V. Murray and K. Hawton, Suicide Life Threat. Behav., 2000, 30(4), 313–326 CAS.
- D. C. Dahlin, G. T. Miwa, A. Y. Lu and S. D. Nelson, Proc. Natl. Acad. Sci. U. S. A., 1984, 81(5), 1327–1331 CrossRef CAS.
- J. Das, J. Ghosh, P. Manna and P. C. Sil, Free Radical Res., 2010, 44(3), 340–355 CrossRef CAS PubMed.
- C. H. Chou, C. W. Liu, D. J. Yang, Y. H. Samuel-Wu and Y. C. Chen, Food Chem., 2015, 168, 63–69 CrossRef CAS PubMed.
- E. D. Guerrero, R. C. Mejias, R. N. Marin, M. P. Lovillo and C. G. Barroso, J. Sci. Food Agric., 2010, 90(4), 712–718 CAS.
- S. Nishidai, Y. Nakamura, K. Torikai, M. Yamamoto, N. Ishihara, H. Mori and H. Ohigashi, Biosci., Biotechnol., Biochem., 2000, 64(9), 1909–1914 CrossRef CAS PubMed.
- N. E. Mohamad, S. K. Yeap, K. L. Lim, H. M. Yusof, B. K. Beh, S. W. Tan, W. Y. Ho, S. A. Sharifuddin, A. Jamaluddin, K. Long, N. M. A. N. A. Rahman and N. B. Alitheen, Chin. Med., 2015, 10, 3 CrossRef PubMed.
- R. Nur Aimi, F. Abu Bakar and M. H. Dzulkifly, Int. Food Res. J., 2013, 20(1), 369–376 Search PubMed.
- N. Prasad, B. Yang, K. W. Kong, H. E. Khoo, J. Sun, A. Azlan, A. Ismail and Z. B. Romli, Evidence-Based Complementary and Alternative Medicine, 2013, 2013, 154606 CrossRef PubMed.
- K. Tsuji, M. N. F. Ghazalli, Z. Ariffin, M. S. Nordin, M. I. Khaidizar, M. E. Dulloo and L. S. Sebastian, Sains Malays., 2011, 40(12), 1407–1412 Search PubMed.
- A. Aziz and R. Jack, J. Sustainability Sci. Manage., 2015, 10(1), 87–91 CrossRef.
- A. A. Shamsuddin, M. Najiah, A. Suvik, M. N. Azariyah, B. Y. Kamaruzzaman, A. W. Effendy and A. B. John, World Appl. Sci. J., 2013, 24(2), 333–340 Search PubMed.
- C. Kong, W. A. Yehye, N. A. Rahman, M. W. Tan and S. Nathan, BMC Complementary Altern. Med., 2014, 14, 4 CrossRef PubMed.
- M. Bakshi and P. Chaudhuri, Int. J. Pharma Bio Sci., 2014, 5(1), 294–304 Search PubMed.
- M. Z. M. Satar, M. W. Samsudin and M. R. Othman, Malaysian Journal of Analytical Sciences, 2011, 15(2), 258–264 Search PubMed.
- J. Kongkiattikajorn, Kasetsart J.: Nat. Sci., 2014, 48, 980–988 CAS.
- T. Marino, A. Galano and N. Russo, J. Phys. Chem. B, 2014, 118(35), 10380–10389 CrossRef CAS PubMed.
- A. Perez-Gonzalez, A. Galano and J. R. Alvarez-Idaboy, New J. Chem., 2014, 38, 2639–2652 RSC.
- M. K. Rasool, E. P. Sabina, S. R. Ramya, P. Preety, S. Patel, N. Mandal, P. P. Mishra and J. Samuel, J. Pharm. Pharmacol., 2010, 62(5), 638–643 CrossRef CAS PubMed.
- C. L. Liu, J. M. Wang, C. Y. Chu, M. T. Cheng and T. H. Tseng, Food Chem. Toxicol., 2002, 40(5), 635–641 CrossRef CAS PubMed.
- B. Singh, A. K. Saxena, B. K. Chandan, S. G. Agarwal and K. K. Anand, Indian J. Physiol. Pharmacol., 2001, 45(4), 435–441 CAS.
- J. Ozer, M. Ratner, M. Shaw, W. Bailey and S. Schomaker, Toxicology, 2008, 245(3), 194–205 CrossRef CAS PubMed.
- M. K. Rasool, E. P. Sabina, S. R. Ramya, P. Preety, S. Patel, N. Mandal, P. P. Mishra and J. Samuel, J. Pharm. Pharmacol., 2010, 62(5), 638–643 CrossRef CAS PubMed.
- X. Wang, Y. Lu and A. I. Cederbaum, Hepatology, 2005, 42(2), 400–410 CrossRef CAS PubMed.
- K. Mandery, K. Bujok, I. Schmidt, M. Keiser, W. Siegmund, B. Balk and H. Glaeser, Biochem. Pharmacol., 2010, 80, 1746–1753 CrossRef CAS PubMed.
- R. V. Priyadarsini and S. Nagini, Free Radical Res., 2012, 46(1), 41–49 CrossRef PubMed.
- I. Klopcic, M. Poberznik, J. Mavri and M. S. Dolenc, Chem.-Biol. Interact., 2015, 242, 407–414 CrossRef CAS PubMed.
- M. Pavlin, M. Repic, R. Vianello and J. Mavri, Mol. Neurobiol., 2015, 53(5), 3400–3415 CrossRef PubMed.
- R. Zamora, Y. Vodovotz and T. R. Billiar, Mol. Med., 2000, 6, 347–373 CAS.
- N. Karunaweera, R. Raju, E. Gyengesi and G. Munch, Front. Mol. Neurosci., 2015, 8, 24 Search PubMed.
- C. Y. He, B. B. Liang, X. Y. Fan, L. Cao, R. Chen, Y. J. Guo and J. Zhao, Acta Pharmacol. Sin., 2012, 33, 1004–1012 CrossRef CAS PubMed.
- J. A. Hinson, D. W. Roberts and L. P. James, Handb. Exp. Pharmacol., 2011, 196, 369–405 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra13409b |
| This journal is © The Royal Society of Chemistry 2016 |