Full vs. partial competitive binding behaviour in molecularly imprinted polymers. The case for a chlorinated phenoxyacids-binding polymer
Abstract
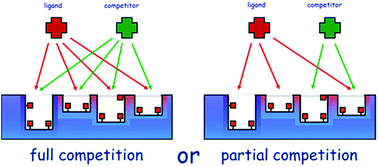
The assessment of the molecular recognition properties of a molecularly imprinted polymer (MIP) towards its target molecule requires the determination of its binding thermodynamic parameters through the measurement of the binding isotherm that governs the binding equilibrium. This measurement is usually performed assuming that the binding properties of a MIP are not affected by the simultaneous presence of two or more ligands of comparable affinity for the binding sites (full competitivity). The validity of this requirement is particularly critical when the selectivity of a MIP is evaluated. In fact, if conditions of full competitiveness fail to exist, the selectivity measured in the presence of one ligand at a time can be markedly different from the selectivity measured in the simultaneous presence of several different ligands. The goal of this work is to test whether the binding parameters of a MIP for its template molecule result are modified by the presence of a competitor of comparable affinity. As template and competitor molecules the herbicides 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) and 2,4-dichlorophenoxyacetic acid (2,4-D) were chosen. The binding data obtained by partition experiments were fitted by using the general Freundlich–Langmuir competitive isotherm model for multiple ligands. The results show that the equilibrium constant (Keq) and the maximum binding site concentration (Bmax) calculated in both conditions are statistically indistinguishable. Thus, it can be concluded that the thermodynamics that govern the binding behavior of a MIP works in full competitive conditions.


 Please wait while we load your content...
Please wait while we load your content...